Abnormal Colligative Properties
The experimental values of colligative properties in most of the cases resemble closely to those obtained theoretically by their formula. However, in some cases experimental values of colligative properties differ widely from those obtained theoretically. Such experimental values are referred to as abnormal colligative properties.
The abnormal behaviour of colligative properties has been explained in terms of dissociation and association of solute molecules.
a. Dissociation of solute molecules
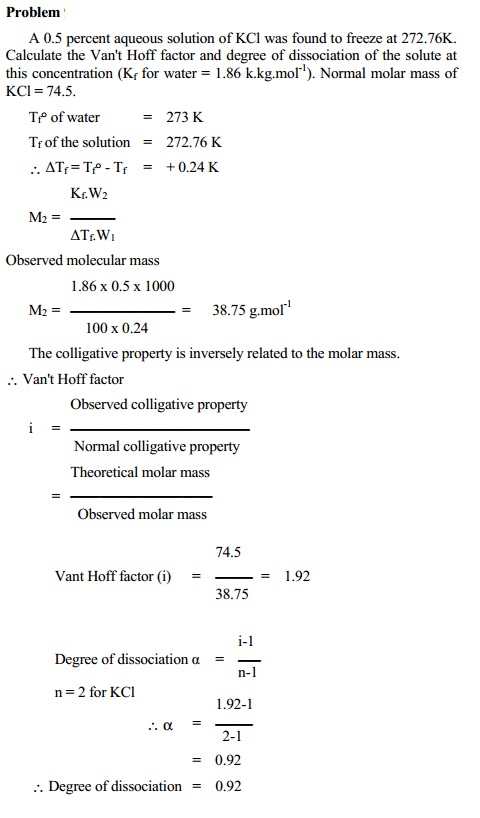
Such solutes which dissociate in solvent (water) i.e. electrolytes, show an increase in number of particles present in solution. This effect results in an increase in colligative properties obtained experimentally.
The Van't Hoff factor (i)
i = Experimental colligative property / Normal colligative property
i > 1 for dissociation. We can calculate the degree of dissociation (a) using the equation.
dissociation = i - 1 / n - 1
where `n' is the total number of particles furnished by one molecule of the solute.
For example, sodium chloride in aqueous solution exists almost entirely as Na+ and Cl- ions. In such case, the number of effective particles increases and therefore observed colligative property is greater than normal colligative property.

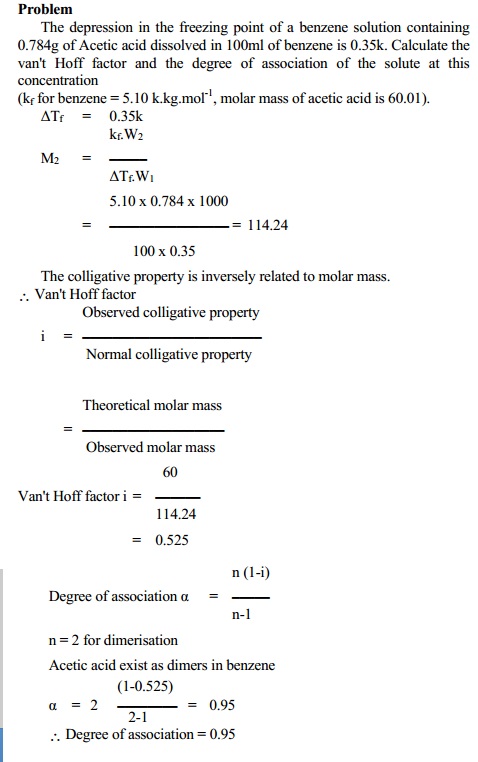
b. Association of the solute molecules
Such solute which associate in a solvent show a decrease in number of particles present in solution. This effect results in a decrease in colligative properties obtained experimentally.
Here,
Experimental Colligative Property < Normal Colligative Property \ Vant Hoff factor
i = Experimental Colligative Property / Normal colligative property
i < 1 for association
Using this, the degree of association 'a' can be calculated from
a(association) = (1-i)n / (n-1)
where `n' is the number of small molecules that associate into a single
larger new molecule.
For example, molecules of acetic acid dimerise in benzene due to intermolecular hydrogen bonding. In this case, the number of particles is reduced to half its original value due to dimerisation. In such case, the experimental colligative property is less than normal colligative property.
2 (CH3COOH) -- > < -- (CH3COOH)2