Surface Energy (Surface Wetting Capability)
As previously mentioned, one of the important characteristics of a liquid penetrant material is its ability to freely wet the surface of the object being inspected. At the liquid-solid surface interface, if the molecules of the liquid have a stronger attraction to the molecules of the solid surface than to each other (the adhesive forces are stronger than the cohesive forces), wetting of the surface occurs. Alternately, if the liquid molecules are more strongly attracted to each other than the molecules of the solid surface (the cohesive forces are stronger than the adhesive forces), the liquid beads-up and does not wet the surface of the part.
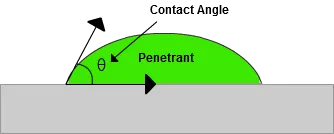
One way to quantify a liquid's surface wetting characteristics is to measure the contact angle of a drop of liquid placed on the surface of an object. The contact angle is the angle formed by the solid/liquid interface and the liquid/vapor interface measured from the side of the liquid. (See the figure below.) Liquids wet surfaces when the contact angle is less than 90 degrees. For a penetrant material to be effective, the contact angle should be as small as possible. In fact, the contact angle for most liquid penetrants is very close to zero degrees.

Wetting ability of a liquid is a function of the surface energies of the solid-gas interface, the liquid-gas interface, and the solid-liquid interface. The surface energy across an interface or the surface tension at the interface is a measure of the energy required to form a unit area of new surface at the interface. The intermolecular bonds or cohesive forces between the molecules of a liquid cause surface tension. When the liquid encounters another substance, there is usually an attraction between the two materials. The adhesive forces between the liquid and the second substance will compete against the cohesive forces of the liquid. Liquids with weak cohesive bonds and a strong attraction to another material (or the desire to create adhesive bonds) will tend to spread over the material. Liquids with strong cohesive bonds and weaker adhesive forces will tend to bead-up or form a droplet when in contact with another material.
In liquid penetrant testing, there are usually three surface interfaces involved, the solid-gas interface, the liquid-gas interface, and the solid-liquid interface. For a liquid to spread over the surface of a part, two conditions must be met. First, the surface energy of the solid-gas interface must be greater than the combined surface energies of the liquid-gas and the solid-liquid interfaces. Second, the surface energy of the solid-gas interface must exceed the surface energy of the solid-liquid interface.
A penetrant's wetting characteristics are also largely responsible for its ability to fill a void. Penetrant materials are often pulled into surface breaking defects by capillary action. The capillary force driving the penetrant into the crack is a function of the surface tension of the liquid-gas interface, the contact angle, and the size of the defect opening. The driving force for the capillary action can be expressed as the following formula:
Force = 2 prs LG cosq
Where:
r = radius of the crack opening (2pr is the line of contact between the liquid and the solid tubular surface.)
s LG = liquid-gas surface tension
q = contact angle
Since pressure is the force over a given area, it can be written that the pressure developed, called the capillary pressure, is
Capillary Pressure = (2 s LG cosq)/ r
The above equations are for a cylindrical defect but the relationships of the variables are the same for a flaw with a noncircular cross section. Capillary pressure equations only apply when there is simultaneous contact of the penetrant along the entire length of the crack opening and a liquid front forms that is an equidistant from the surface. A liquid penetrant surface could take-on a complex shape as a consequence of the various deviations from flat parallel walls that an actual crack could have. In this case, the expression for pressure is
Capillary Pressure = 2(s SG - ss SL)/r = 2S /r
Where:
s SG = the surface energy at the solid-gas interface.
s SL = the surface energy at the solid-liquid interface.
r = the radius of the opening.
S = the adhesion tension (sSG - s SL).
Therefore, at times, it is the adhesion tension that is primarily responsible for a penetrant's movement into a flaw and not the surface energy of the liquid-gas interface. Adhesion tension is the force acting on a unit length of the wetting line from the direction of the solid. The wetting performance of the penetrant is degraded when adhesion tension is the primary driving force.
It can be seen from the equations in this section that the surface wetting characteristics (defined by the surface energies) are important in order for a penetrant to fill a void. A liquid penetrant will continue to fill the void until an opposing force balances the capillary pressure. This force is usually the pressure of trapped gas in a void, as most flaws are open only at the surface of the part. Since the gas originally in a flaw volume cannot escape through the layer of penetrant, the gas is compressed near the closed end of a void.
Since the contact angle for penetrants is very close to zero, other methods have been devised to make relative comparisons of the wetting characteristics of these liquids. One method is to measure the height that a liquid reaches in a capillary tube. However, the solid interface in this method is usually glass and may not accurately represent the surface that the penetrant inspection will be performed on. Another method of comparative evaluation is to measure the radius, the diameter, or the area of a spot formed when a drop of penetrant is placed on the test surface and allowed to stand undisturbed for a specific amount of time. However, using this method, other factors are also acting in the comparison. These methods include the density, viscosity, and volatility of the liquid, which do not enter into the capillarity equations, but may have an effect on the inspection as discussed in the related pages.