Combustion of Fuels

The combustion of fuels may be defined as a chemical combination of oxygen, in the atmospheric air, and hydro-carbons. The following chemical equations for the chemical combination of oxygen (representing combustion) are important :
1. When carbon burns in sufficient quantity of oxygen, carbon dioxide is produced along with a release of large amount of heat. This is represented by the following chemical equation :

It means that 1 kg of carbon requires 8 / 3 kg of oxygen for its complete combustion, and produces 11 / 3 kg of carbon dioxide gas.
2. If sufficient oxygen is not available, then combustion of carbon is incomplete. It then produces carbon monoxide instead of carbon dioxide. It is represented by the following chemical equation:

It means that 1 kg of carbon requires 4 / 3 kg of oxygen, and produces 7 / 3 kg of carbon monoxide.
3. If carbon monoxide is burnt further, it is converted into carbon dioxide. Thus

It means that 1 kg of carbon monoxide requires 4 7 kg of oxygen, and produces 11 / 7 kg of carbon dioxide.
4. When sulphur burns with oxygen, it produces sulphur dioxide. This is represented by the following chemical equation

It means that 1 kg of sulphur requires 1 kg of oxygen for complete combustion to produce 2 kg of sulphur dioxide.
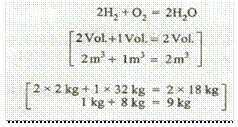
It means that 2 volumes of hydrogen require 1 volume of oxygen to produce 2 volumes of water or steam.
Or in other words, 1 kg of hydrogen requires 8 kg of oxygen and produces 9 kg of water or steam.

It means that 1 volume of methane requires 2 volumes of oxygen and produces 1 volume of carbon dioxide and 2 volumes of water or steam.
Or in other words, 1 kg of methane requires 4 kg of oxygen and produces 11 / 4 kg of carbon dioxide and 9 / 4 kg of water or steam.
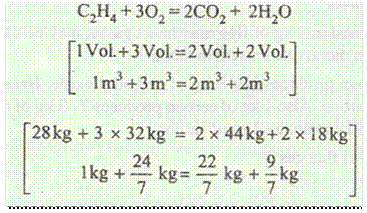
It means that 1 volume of ethylene requires 3 volumes of oxygen and produces 2 volumes of carbon dioxide and 2 volumes of water or steam.
Or in other words, 1 kg of ethylene requires 24 / 7 kg of oxygen and produces 22 / 7 kg of carbon dioxide and 9 / 7 kg of water or steam.