Constant Pressure (Isobaric) Process
Constant Pressure or Isobaric Process - When the gas is heated at a constant pressure, its temperature and volume will increase. Since there is a change in its volume, the heat supplied is utilized in increasing the internal energy of the gas, and also for doing some external work. It may be noted that this process is governed by Charles' law.
Now consider m kg of a certain gas being heated at a constant pressure from an initial temperature T1 to a final temperature T2. This process is shown on the p-v diagram in Fig. 5.2.

We know that heat supplied to the gas at constant

Increase in internal energy,
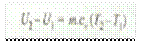
and work done during the process or by the gas,

Notes:
(a) When the gas is cooled at constant pressure, there will be a compression. The temperature and volume will decrease during cooling and work is said to be done on the gas. In this case,
Heat rejected by the gas,
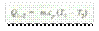
Decrease in internal energy,
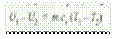
and workdone on the gas,
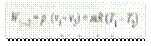
(b) During expansion or heating process, work is done by the gas (i.e. W1-2 is +ve); internal energy of the gas increases (i.e. dU is +ve) and heat is supplied to the gas (i.e. Q1-2 is +ve).
(c) During compression or cooling process, work is done on the gas (i.e. W1-2 is -ve); internal energy of the gas decreases (i.e. dU is —ve) and heat is rejected by the gas (i.e. Q1-2 is -ve).