Thermodynamic System
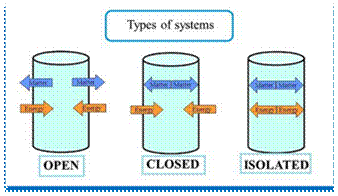
The thermodynamic system may be defined as a definite area or a space where some thermodynamic process takes place. It may be noted that a thermodynamic system has its boundaries, and anything outside the boundaries is called its surroundings. The thermodynamic system may be classified into the following three groups.
1. Closed system. This is a system of fixed mass whose boundaries are determined by the space of the working substance occupied in it. In a closed system, heat and work cross the boundary of the system, but there is no addition or loss of the original mass of the working substance. Thus the mass of the working substance which comprises the system, is fixed.
2. Open system. In this system, the working substance crosses the boundary of the system. The heat and work may also cross the boundary.
3. Isolated system. It is a system of fixed mass and no heat or work cross its boundary.