Universal Gas Constant
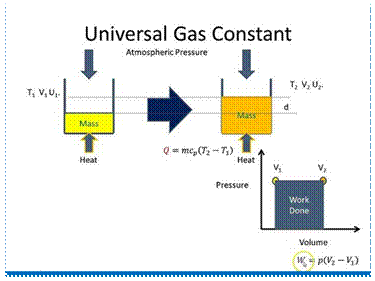
The universal gas constant or molar constant (generally denoted by Ru) of a gas is the product of the gas constant and the molecular mass of the gas. Mathematically,
Ru = MR
where
M = Molecular mass of the gas expressed in kg-mole, and
R = Gas constant
In general, if M1, M2, M3, etc. are the molecular masses of different gases and R1, R2, R3, etc. are their gas constants respectively, then
M1R1 = M2R2= M3R3= ...= Ru
Notes:
1. The value of Ru is same for all gases.
2. In S.I. units, the value of Ru is taken as 8314 J / kg-mole K or 8.314 kJ / kg-mole K.
3. The characteristic gas equation (i.e. pv = RT) may be written in terms of molecular mass as: pv = MRT