Joule's Law
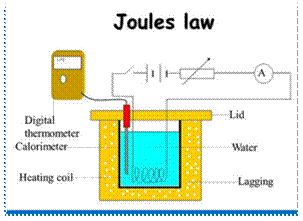
Joule's Law states, "The change of internal energy of a perfect gas is directly proportional to the change of temperature." Mathematically,

where
m = Mass of the gas, and
c = A constant of proportionality, known as specific heat.
Note: From the Joule's law, we see that whenever a gas expands, without doing any external work and without taking in or giving out heat, its internal energy as well as temperature does not change.