Classification of Thermodynamic Cycles
The thermodynamic cycles are classified into the following groups:
1. Reversible cycle.
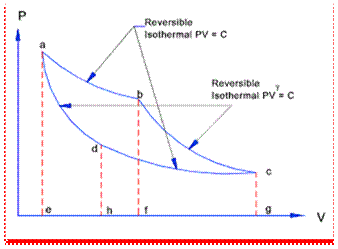
A process, in which some change in the reverse direction, reverses the process completely, is known as a reversible process. In a reversible. process there should not be any loss of heat due to friction, radiation or conduction, etc. A cycle will be reversible if all the processes constituting the cycle are reversible. Thus in a reversible cycle, the initial conditions are restored at the end of the cycle.
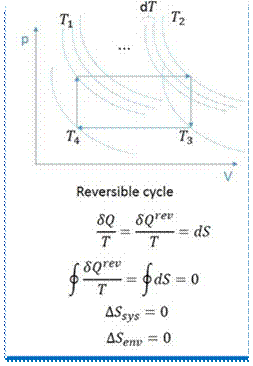
A little consideration will show, that when the operations are performed in the reversed order, the cycle draws heat from the cold body and rejects it to the hot body. This operation requires an external power to drive the mechanism according to second law of thermodynamics. A machine which operates on a reversed cycle is regarded as a "heat pump", such as a refrigerator, because it pumps heat from the cold body to the hot body. Following are the conditions for reversibility of a cycle:
1. The pressure and temperature of the working substance must not differ, appreciably, from those of the surroundings at any stage in the process.
2. All the processes, taking place in the cycle ,of operation, must be extremely slow.
3. The working parts of the engine must be friction free.
4. There should be no loss of energy during the cycle of operation.
Note: A reversible cycle should not be confused with a mechanically reversible engine. Steam engine cranks may be made to revolve in a reversed direction by mechanically altering the valve settings. But this does not reverse the cycle, on which it works. A two-stroke petrol engine may be made to revolve in reverse direction by altering the timing of ignition. But this also does not reverse the actual cycle.
2. Irreversible cycle.
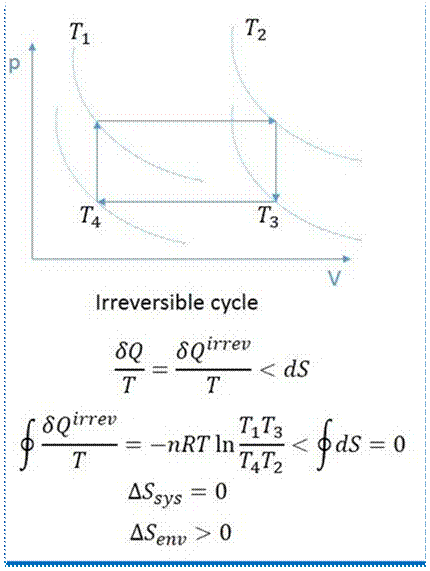
A process, in which change in the reverse direction, does not reverse the process, is called irreversible process. In an irreversible process, there is a loss of heat due to friction, radiation or conduction,
In an actual practice, most of the processes are irreversible to some degree. The main causes for the irreversibility are
(i) mechanical and fluid friction,
(ii) unrestricted expansion
(iii) heat transfer with a finite temperature difference.
Moreover, friction converts the mechanical work into heat. This heat cannot supply back the same amount of mechanical work, which was consumed for its production. Thus, if there is some friction involved in the process, it becomes irreversible. A cycle will be irreversible if any of the processes, constituting the cycle, is irreversible. Thus in an irreversible cycle, the initial conditions are not restored at the end of the cycle.
Notes:
1. We have discussed the various thermodynamic processes. The processes such as constant volume, constant pressure, isothermal or constant temperature (i.e. p.v = C), adiabatic and polytropic are all reversible processes.
2. The throttling is an irreversible process.