CONDUCTION
1) Conduction heat transfer is due to random molecular and atomic vibrational, rotational and translational motions
a) High temperature and more energetic molecules vibrate more and transfer energy to less energetic particles as a result of molecular collisions or interactions
2) The heat flux (a
vector) 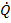 is characterized by a
transport property know as the
is characterized by a
transport property know as the
a) Thermal Conductivity, k (W / m · K)
b) W = watts m = Meters K = temperature in Kelvin
3) Conduction is the transfer of energy from the more energetic particles of a substance to the adjacent less energetic ones as a result of interactions between the particles.
4) Conduction can take place in solids, liquids, or gases. In gases and liquids, conduction is due to the collisions and diffusion of the molecules during their random motion. In solids, it is due to the combination of vibrations of the molecules in a lattice and the energy transport by free electrons
5) The rate of heat conduction through a medium depends on the rate of heat conduction through a medium depends on the geometry of the medium, its thickness, and the material of the medium, as well as the temperature difference across the medium
i) 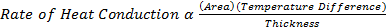
6) Fourier’s Law
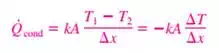
In limiting case of Δx →0
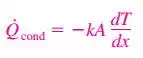
a) The negative sign in Eq. ensures that heat transfer in the positive x direction is a positive quantity.
b) The heat transfer area A is always normal to the direction of heat transfer.
c) k is Thermal Conductivity, Units W/m·K or W/m.°C
i) Thermal conductivity of a material can be defined as the rate of heat transfer through a unit thickness of the material per unit area per unit temperature difference.
ii) The thermal conductivity of a material is a measure of the ability of the material to conduct heat. A high value for thermal conductivity indicates that the material is a good heat conductor, and a low value indicates that the material is a poor heat conductor or insulator. For example, k = 0.608 W/m·°C for water and k = 80.2 W/m·°C for iron at room temperature, which indicates that iron conducts heat more than 100 times faster than Water can.
iii) The thermal conductivities of gases such as air vary by a factor of 104 from those of pure metals such as copper.
iv) Note that pure crystals and metals have the highest thermal conductivities and gases and conductivities, and gases and insulating materials the lowest.
v) The thermal conductivity of a substance is normally highest in the solid phase and lowest normally highest in the solid phase and lowest in the gas phase.
vi) Unlike gases, the thermal conductivities of most liquids decrease with increasing temperature, with water being a notable exception
vii) In solids, heat conduction is due to two effects: the lattice vibrational waves induced by the vibrational motions of the molecules positioned at relatively fixed positions in a periodic manner called a lattice, and the energy transported via the free flow of energy transported via the free flow of electrons in the solid.
(1) The thermal conductivity of a solid is obtained by adding the lattice and electronic components the relatively high thermal conductivities and electronic components. The relatively high thermal conductivities of pure metals are primarily due to the electronic component.
(2) The lattice component of thermal conductivity strongly depends on The lattice component of thermal conductivity strongly depends on the way the molecules are arranged
(3) Unlike metals, which are good electrical and heat conductors, crystalline solids such as diamond and semiconductors such as silicon are good heat conductors but poor electrical conductors. As a result, such materials find widespread use in the electronics industry. Despite their higher price, diamond heat sinks are used in the cooling of sensitive electronic components because of the excellent thermal conductivity of diamond. Silicon oils and gaskets are commonly used in the packaging of electronic components because they provide both good thermal contact and good electrical insulation.
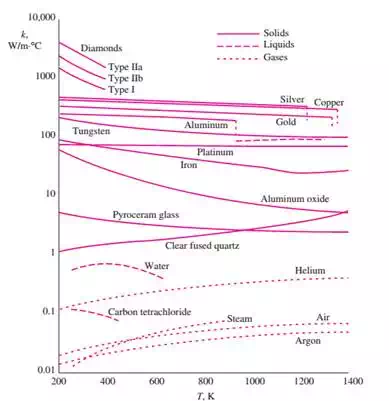
Figure. The variation of the thermal conductivity of various solids, liquids, and gases with temperature
7) Thermal Diffusivity: Material property that appears in the transient heat conduction analysis is the thermal diffusivity, which represents how fast heat diffuses through a material and is defined as

a) The product ρCp, which is frequently encountered in heat transfer analysis, is called the heat capacity of a material
b) The larger the thermal diffusivity, the faster the propagation of heat into the medium. A small value of thermal diffusivity means that heat is mostly absorbed by the material and a small amount of heat will be conducted further.