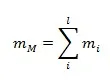
MASS AND MOLE FRACTION
If we want to deal with the problems of gas mixture, two laws should always be taken into consideration. One is the conservation of mass. And the other is the conservation of moles for a nonreacting mixture.
Assuming that a gas mixture consists of components
According to the conservation of mass, we have:
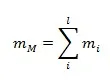
According to the conservation of moles for a nonreacting mixture, we have:
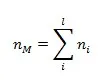
(this equation is only available for a nonreacting mixture!)
The subscript “i” denotes the components; the subscript “M” denotes the mixture. The number of components is “l”.
For example, we have two containers. One is filled with H2 of mH2=15kg. The other is filled with He of mHe=30kg. If we mix both gases in a third container, then the mass of gas mixture in this third container is mM= mH2+ mHe (= 45kg in this case).
Similarly, we have 1mol H2 and 2 mol He. Then the number of moles is the sum of the number of moles of its components. In this case we obtain a gas mixture of nM=3 mol.
In additional, we have mass fraction ξ and mole fraction γ:
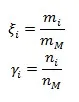
And it is obvious that the sum of mass fraction and of mole fraction equals to unity:

Since we now obtain these both fractions, we can calculate the following two useful values:
Average molar mass:
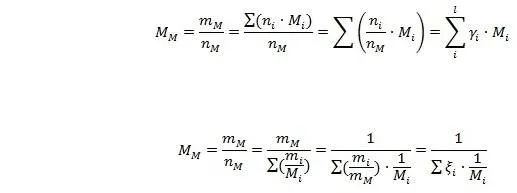
Average gas constant of the mixture:
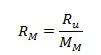
where Ru is universal gas constant = 8,314J/(mol·K)