Fluids are divided into five types they are:
- Ideal fluids
- Real fluids
- Newtonian fluids
- Non-Newtonian fluids
- Ideal plastic fluids
Ideal Fluids:
An ideal fluid is defined as a fluid which is in-compressible and the one that does not have viscosity. Ideal fluids are imaginary fluids. This exist some viscosity.
Real Fluids:
A real fluid is defined as a fluid which possesses viscosity. In actual state all fluids are real fluids.
Newtonian fluids:
In a real fluid the shear stress is directly proportional to the velocity gradient or shear strain, which is known as Newtonian fluids.
Non-Newtonian fluids:
In a real fluid the shear stress is not proportional to the velocity gradient or shear strain, which is known as Non- Newtonian fluids.
Ideal plastic fluids:
In a fluid the shear stress is more than the yield value. The shear stress is proportional to the rate of velocity gradient or shear strain is known as ideal plastic fluids.
Thermodynamic properties:
Fluid consists of either liquid or gases. In case of gases they are compressible fluids. The thermodynamic properties play an important key role. Due to the change in temperature and pressure the gases undergoes high variation in densities. So the relation between the absolute temperature, absolute pressure and specific volume is
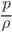 = RT
= RT
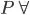 = RT
= RT
P= Absolute pressure of a gas
R = gas constant
T= Absolute temperature in kelvin
ρ = Density of a gas.

R Dimension
The gas constant R value must be depends on the particular gas.
In MKS Unit R value is 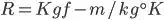
In SI units 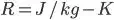
Isothermal Process:
At constant pressure the change in density occurs then the process is known as isothermal process. The relation between pressure and density is:
p/ρ= constant
Adiabatic process:
The change in the density must be occurs without heat change to and fro the gases is known as Adiabatic process.
Due to friction there is no heat generation in the gases, so the relation between the density and pressure is
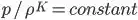
K value for air is 1.4
Universal gas constant:
It is also known as gas constant, ideal gas constant, molar gas constant. It is denoted by the letter R.
In SI units R value is 8314 J/kg-mole.
Compressibility and bulk modulus:
The reciprocal of bulk modules of elasticity is known as compressibility. It is defined as the ratio of compressive stress to volumetric strain.
Consider a cylinder and piston is inserted into it, when the force is applied on the piston then the pressure is increased to p+dp. Then the volume present in the cylinder is decreased to 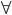 to
to 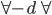 .
.
Volumetric strain must be =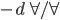
Bulk modules = K = increase in pressure / volumetric strain
Compressibility = 1/ K
For gases relation between Bulk modulus and pressure:
K=pk