Pig iron and its manufacturing

Pig iron is the crude form of iron and is used as a raw material for the production of various other ferrous metals, such as cast iron, wrought iron and steel. The pig iron is obtained by smelting iron ores in a blast furnace.
The iron ores are found in various forms as shown below:
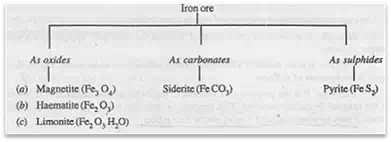
The metallic contents of these iron ores are given in the following table:

The haematite is widely used for the production of pig iron. Since pyrite contains only 30 to 40% iron, therefore it is not used for manufacturing pig iron.
The pig iron is obtained from the iron ores in the following steps:
1. Concentration. It is the process of removing the impurities like clay, sand etc. from the iron ore by washing with water.
2. Calcination or roasting. It is the process of expelling moisture, carbon dioxide, sulphur and arsenic from the iron ore by heating in shallow kilns.
3. Smelting. It is process of reducing the ore with carbon in the presence of a flux. The smelting is carried out in a large tower called blast furnace.
The blast furnace is a chimney like structure made of heavy steel plates lined inside with fire bricks to a thickness of 1.2 to 1.5 metres. It is about 30 metres high with a maximum internal diameter of 9 meters as its widest cross-section. The portion of the furnace above its widest cross-section is called stack. The top most portion of the stack is called throat through which the charge is fed into the furnace. The charge of the blast furnace consists of calcined ore (8 parts), coke (4 parts) and lime stone (1 part). The portion of the furnace, below its widest cross-section is known as bosh or the burning zone (or zone of fusion). The bosh is provided with holes for a number of water jacketed iron blowing pipes known as tuyers. The tuyers are 12 to 15 in number and are connected to bustle pipe surrounding the furnace.
In the lower part of the blast furnace (called zone of fusion), the temperature is 1200° C to 1300° C. In the middle part of the blast furnace (called zone of absorption), the temperature is 800° C to 1000°C. In the upper part of the blast furnace (called zone of reduction), the temperature is 400° C to 700° C.
At the bottom of the blast furnace, the molten iron sinks down while above this floats the fusible stage which protects the molten iron from oxidation. The molten iron thus produced is known as pig iron. The slag from the blast furnace consists of calcium, aluminum and ferrous silicates. It is used as a ballast for rail roads, mixed with tar for road making and in the cement manufacture.
The pig iron from the blast furnace contains 90 to 92% of iron. The various other elements present in pig iron are carbon (1 to 5%), silicon ( 1 to 2%), manganese (1 to 2%), sulphur and phosphorus (1 to 2%).
Note : Carbon plays an important role in iron. It exists in iron in two forms i.e. either in a free form (as graphite) or in a combined form (as cementite and pearlite). The presence of free carbon in iron imparts softness and a coarse crystalline structure to the metal, while the combined carbon makes the metal hard and gives a fine grained crystalline structure.