Ocean density
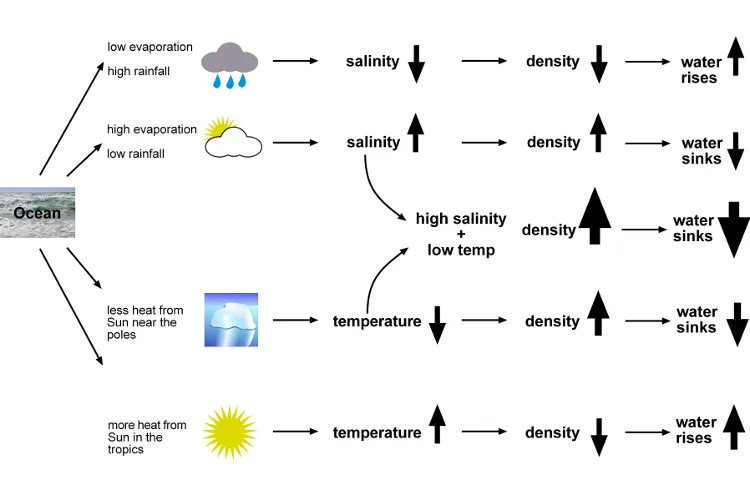
The density of seawater plays a vital role in causing ocean currents and circulating heat because of the fact that dense water sinks below less dense. Salinity, temperature and depth all affect the density of seawater.
Density is a measure of how tightly a certain amount of matter is packed into a given volume. The more the stuff is packed in, the higher the density. Density can be calculated by dividing an object’s mass by its volume. It is commonly measured in grams per millilitre or grams per cubic centimetre.
For example, basalt is a very dense rock with an average mass of around 3 g/cm3 but it is still not as dense as elemental iron, with a mass of nearly 8 g/cm3.
Big ocean currents
Professor Keith Hunter describes how the North Atlantic current, and others like it, flush out and ventilate the world’s oceans. Because of the size of the ocean, and the slow speed of the currents, water that sinks in the North Atlantic may not see the surface again for a thousand years.
Seawater is not just water – it has lots of chemicals packed into it. This means it is denser than pure water. The higher the salinity, the higher the density.
Seawater density varies from place to place because it is affected by salinity and temperature. This means that ships float higher or lower in the water, depending on the density of the ocean. If you look near the waterline of a cargo ship, you should find the International Load Line, once called the Plimsoll Line. This shows the limit of where the fully loaded ship should sit in waters of different densities.

High salinity makes water denser. This is because there is more salt packed into the water.
High temperature makes water less dense. As water gets warmer, its molecules spread out, so it becomes less dense. As it gets colder, it becomes denser. Most chemicals get denser when they turn from a liquid to a solid, but water is different. When liquid water freezes into solid ice, it becomes less dense. When ice forms, water molecules arrange themselves into a rigid but open pattern. This structure is less dense than the liquid water, so ice floats.
Deep water is denser than shallow water. The water molecules are packed together more tightly because of the weight of water above pushing down.