Fabrication and Properties of Zinc Composite Coatings for Mitigation of Corrosion in Coastal and Marine Zone
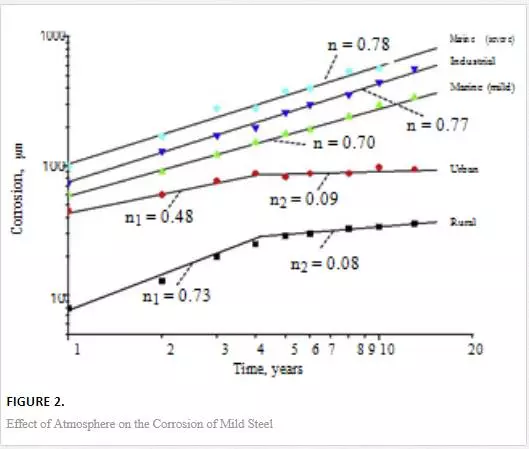
Metallic materials and structures (mainly steel) exposed to marine and coastal environments are constantly at risk of corrosion, which compromises their structural integrity and life span. Deterioration of materials due to corrosion can also have other serious implications such as economic costs, safety and environmental issues. The corrosion of these materials is induced by atmospheric pollutants mainly chloride ions. According to [1] the atmospheric salinity is dependent mainly on the distance from the sea shore (concentration of chloride salts is high near the shore and reduces with increasing distance from the shore) and wind. The salinity in marine and coastal atmosphere is caused by wind which stirs up and entrains sea water. The generated marine aerosol droplets are transported few hundred of meters inland by the same mechanism. Other factors that affect salinity include latitude, ruggedness of the coastline and undulation of the land surface. A high level of salinity is associated with accelerated corrosion rates and formation of unstable corrosion products [2]. Therefore, metallic structures found in marine and coastal environments have higher corrosion rates than those found inland (since chloride concentration is high close to the sea). Despite the magnitude of corrosion in the respective environments, prevention and combating corrosion remain critical to improve the service life of materials. Figures 1 and 2 show surface degradation induced by marine corrosion and the effect of atmosphere on the corrosion of low carbon steel.

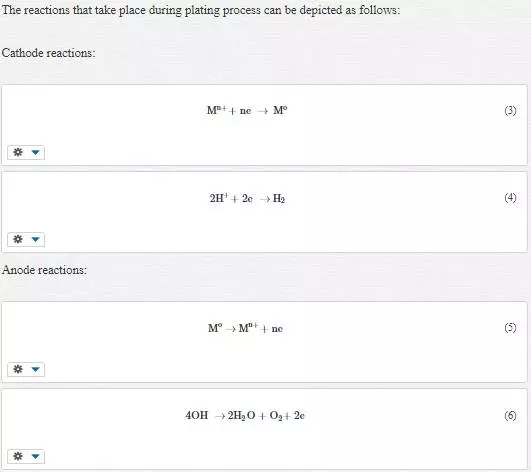
Surface modification and enhancement of properties of the base metal by applying protective coatings has played a significant role in the fight against corrosion. Coatings offer protective barrier between the corrosive environment and the substrate [3]. There are various types of protective coatings that range from nickel, copper, zinc, aluminium, etc., but this chapter focuses only on zinc coatings. Zinc coatings have been successfully used over the past decades to mitigate the corrosion deterioration of mild steel due to their good anti-corrosion properties, ease of fabrication and affordability. Zinc is more active than iron or steel and, thus, offers sacrificial protection to the base metal [4]. Several industries employ extensively this metal for protecting steel used in manufacturing of components, parts or structures against chemical or electrochemical degradation in rural, urban, industrial, coastal and marine atmospheric conditions [5]. However, the exposure of these coatings to aggressive conditions such as industrial, coastal and marine environments induces formation of white rust [6]. Basic reactions that take place during the corrosion of zinc are shown in Equations 1 and 2.
Anodic reaction:

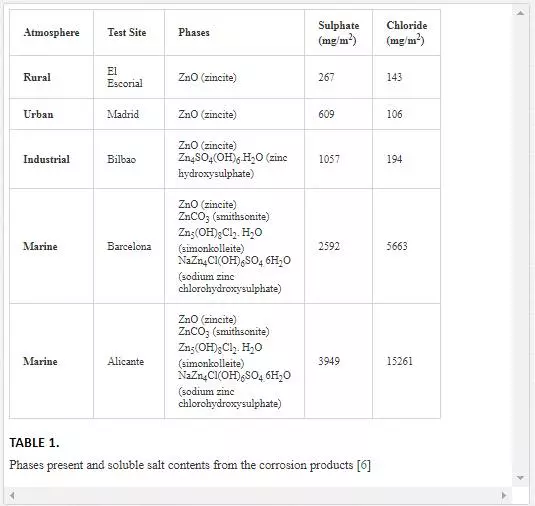
Zinc oxides and hydroxides are commonly the main constituents in the corrosion products which form as a result of corrosion and tend to act as a passivation layer preventing further damage. However, the chemistry of the corrosion products depends on the level and nature of pollutants in the environment. The constituents of the corrosion products in various atmospheres are presented in Table 1. The presence of these atmospheric pollutants (chlorides, carbonates and sulphates) also promotes dissolution of the protective film and thus exposing the fresh metal for further deterioration. Other factors contributing to the degradation of zinc include time of exposure and wetness, pH, stability of corrosion products, relative humidity and erosion [6-8].
To make zinc-based coatings to be strong and durable during application in harsh conditions, metals that are more chemically stable than the matrix are incorporated. Several metals such as aluminium, nickel, copper, cobalt, magnesium and manganese are added to fabricate zinc alloy coatings. These additions promote the evolution of uniform microstructure with limited surface defects and formation of stable and adherent passivation film that can withstand further attack. Many researchers have studied these coatings and found out that the coatings possessed not only excellent corrosion when compared to their traditional counterparts but also good mechanical properties [9-11]. Therefore, the incorporation of these elements into the coatings also extends their applications. The good performance of the coatings has made them to be substitute of cadmium sacrificial coatings and reduce the usage of organic chelating agents and chrome passivation that are not environmentally friendly [12-13]
The ever changing and demanding industry calls for further advancement in the quality of the zinc coatings to cater for the new or improved applications. Exploration, investigation and development of new materials therefore remain critical to finding viable solutions for counteracting the change and demand. Metal oxides, carbides, nitride and so on have been codeposited with Zn to fabricate high corrosion, thermal and wear-resistant composite coatings. The incorporation of these particles into a metal matrix can be accomplished in micro, semi-micro and nano sizes. The composite dispersed particles in micro and semi micro size produced porous structured coatings which have limited corrosion control application. In search for more advanced materials that can cater for these limitations, nano-sized particles were found to have a potential that can eradicate the drawbacks associated with micro-sized particles [14].
Inclusion of nano-sized materials into a Zn matrix to produce nanocomposite coatings offers novel properties that are not exhibited by traditional coatings. The exotic properties such as excellent corrosion resistance, high temperature oxidation resistance, excellent self-lubricating and tribological properties possessed by these materials enable their extensive use for several applications [15]. The incorporation of the second phase particles into the matrix enable excellent performance of the resultant deposits in demanding environments such as coastal and marine. Nanoparticles of metal oxides, carbides, nitrides, borides, etc., have been used as bath additives to improve the properties of Zn matrix. The nanoparticles include Al2O3, TiO2, CeO2, SiO2, SiC, Si3N4, TiN, TiB2, etc.
Electrodeposition is one of the most important surface engineering techniques that is used for fabrication of Zn composite/nanocomposite coatings. This technique exhibits several advantages over the other deposition techniques. The advantages include low cost, versatility, simplicity of operation, high production rates and few size and shape limitations. The quality/performance of the nanocomposite coatings produced by this process depend on a number of operating parameters that include particle concentration in the bath, pH, temperature, current and bath agitation speed. These parameters need to be optimized to achieve the desirable coatings quality [16].Fabrication and study of the properties of zinc composite/nanocomposite coatings using electrodeposition technique have not been thoroughly reviewed in literature. Therefore, this chapter focuses on providing ample information and in-depth analysis of the electrodeposition process (mechanism of incorporation of particles into a metal matrix and optimization of process parameters) and properties (morphological and structural characteristics, hardness, corrosion and wear) of the resultant deposits. This will help zinc electroplaters and corrosion engineers to understand zinc composite/nanocomposite electrodeposition to enable them to produce high quality coatings with improved functional properties.
Fabrication of zinc alloy/composite coatings
Application of coatings on metal surfaces that are prone to corrode is one of the most versatile methods that are employed to protect the metal from degradation. A coating acts as a physical barrier between the corrosive environment and metal, thus providing protection to the substrate from chemical deterioration. In tribological applications, the coating also offers load bearing capabilities. To ensure effective coating performance, the coating must be compatible with the substrate, have good adhesion and possess low porosity. Incorporation of reinforcement nanomaterials into the primary matrix to form composite coating enables production of coatings with advanced properties and good performance during application.There are several techniques available for fabrication of composite coatings. These include, but not limited to, hot-dipping, thermal spraying, sol gel, vapour deposition, laser cladding, and electro- and electroless plating. The coatings resulting from these techniques exhibit different properties and find use in various applications.
HOT DIPPING
Hot-dip galvanizing is a deposition technique for coating iron or steel substrate with zinc by immersing the material of interest in a zinc molten bath [5]. This technique is a batch process and is mainly used on heavy structural steel sections. Galvanizing can be found in almost every major application and industry where iron or mild steel are employed. The surface of the steel requires preparation prior to deposition, and the preparation process includes the following steps:
1. Oil and grease are cleaned from the steel mostly with warm caustic soda to ensure proper chemical interaction between the substrate and molten zinc.
2. The steel is then pickled in dilute solution of hydrochloric or sulphuric acid to remove rust and scale.
3. The steel is immersed in a fluxing bath containing ammonium chloride.
4. The fluxed large sections of steel are then directly dipped into the zinc molten bath for coating.
5. The hot-dip galvanized steel can sometimes be further treated by centrifuging method to reduce the excess zinc on the steel.
Coatings on small components, wires, tubes and sheets can be applied using continuous galvanizing process. The coatings produced by continuous galvanizing process are thinner than that of the batch hot dipping [17]. The bonding of the coating produced by hot dipping is promoted by the interaction of the molten zinc and iron in the steel to form a series of Zn–Fe alloy [18]. The simplicity of galvanizing process enables wide range of applications in protection of steel more than other deposition techniques. The automotive industry is one of the industries that mostly depended on this process. The galvanized sheets are used for the assembling of the body of the car. Other automotive parts are also galvanized to protect them from corrosion attack [19].
THERMAL SPRAYING
Thermal spraying involves the melting and propulsion of metal particles onto a desired substrate using a spraying gun. The material (feedstock) which is to be applied as a coating is fed through the nozzle in a form of powder, wire or rod. It is fed with a stream of gas or air and is melted by a suitable gas-oxygen mixture. The melted material is propelled towards the surface to be coated and forms a consolidated coating on impact [17]. The three main variables that govern this process include the temperature of the flame, the velocity of the particles that are sprayed onto the work piece to fabricate a coating and the nature of the material used to form the coating. There are several types of thermal spraying, which include flame or torch, electric arc and plasma spraying. Thermal sprayed coatings can be made to have good adhesion, controlled thickness and can be applied to existing structures. Surface preparation is required to achieve good adhesion. Coatings fabricated by this technique possess strong bond with the substrate due to the interaction between the coating material and the steel. However, the fabrication of coatings using this technique requires high temperatures and the interaction between the substrate and the coating material makes it difficult to refurbish them when they are worn out.
LASER CLADDING
Laser cladding is another technique that is used to fabricate composite coatings on metal surfaces. This high energy technique employs laser beam to create a melt pool on the surface of the metal substrate in which the reinforcement powder is injected simultaneously. Coatings formed using laser cladding technique are dense and are employed in applications where good wear, corrosion and other surface-related properties are required. Laser cladding also has the capability to control composition and properties within a fabricated structure by either pre-blending or combining different elemental powders using multiple feeder systems at the laser focal zone, which enables tailoring of properties suited for specific engineering applications [20]. The use of laser in coatings technology is costly due to the high energy demand but still finds a variety of use in applications where durability and strength are required.
ELECTRODEPOSITION
Electrodeposition is a technique that is used to apply adherent metallic coating on metal substrate by passing current between two electrodes immersed in an aqueous electrolyte containing ions of the metal of interest causing the metal ions to be deposited on the cathode. The anode is caused to be dissolved to replenish the metal ions in solution by flowing of current and make the cathode to be covered with the deposited metal [21]. Figure 3 shows a schematic diagram of a typical electrolytic cell used for the electrodeposition.
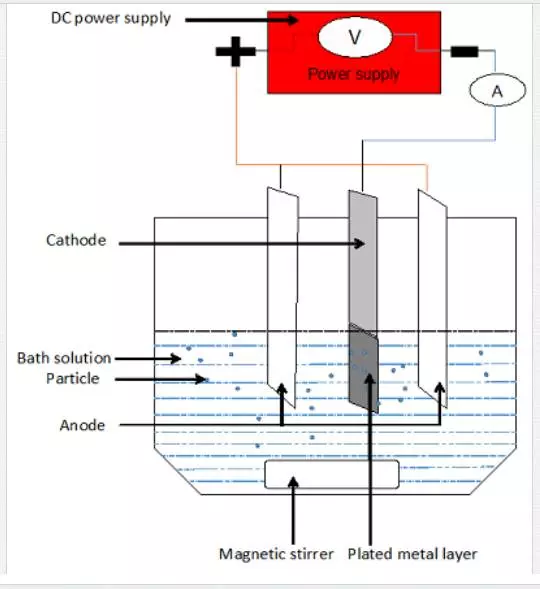
The quality of coatings produced by this technique depends on a number of operating parameters that include the bath composition, throwing power, temperature and bath agitation. Coatings exhibiting different properties such as microstructure, corrosion resistance, adhesion, thickness, hardness, thermal stability and wear resistance can be produced by manipulating these parameters. The flexibility of the electrodeposition enables the technique to find wide variety of applications in mitigation and control of corrosion mainly for small components. However, application of these coatings on large metal structures is beyond the capabilities of this process [21].
Electrodeposited coatings were primarily used for corrosion resistance and decoration (metal finishing). However, industrial technological advancement demand coatings that exhibit functional properties such as wear resistance, electrical properties, magnetic properties, solderability and thermal stability [22]. Zn electrodeposits are constantly improved to cater for this demand and electrodeposition technique makes coatings possible and simple at a lower cost compared to other deposition techniques.
ELECTROLESS PLATING
Electroless plating is a deposition technique used for coating metal bases by auto-catalytic or chemical reduction of metal ions without the application of external current. The internal current required for deposition is provided by the oxidation of reducing agents present in the solution. The quality of the coatings produced by this process makes it to gain more advantage over electrodeposition technique. The coatings exhibit excellent corrosion and wear resistance properties. The desired quality can be attained by controlling operating parameters such as temperature, pH and the composition of the bath. The bath constituents involved in electroless plating include metal ions source, reducing agent, buffering agent, complexing agent and wetting agent [23]. One of the most important bath constituent is a reducing agent since it supplies electrons to reduce metal ions to enable deposition. There are several available reducing agents, which include sodium hypophosphates, amino boranes, sodium borohydrides and hydrazines. Amongst them, sodium phosphates are commonly used due to lower costs and better corrosion resistance of the resulting coating. The reactions involved during electroless plating are as follows:
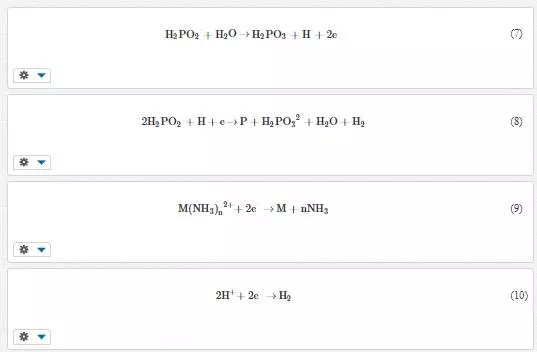
Electroless nickel coatings has assumed the greatest commercial importance among electroless coating. These coatings possess good surface properties, and incorporation of second phase particles enhances the characteristics of the resultant deposits. The second phase particles codeposited with the nickel include silicon carbide, aluminium oxide, carbon nanotubes, etc. The dispersion of these particles requires good bath stability, agitation, particle size, particle concentration and surfactants. This plating technique is complex, requires many additives, operates at elevated temperatures, contain phosphorus or borine and applicable for mostly nickel coatings.
Composite electrodeposition
MECHANISM OF PARTICLE INCORPORATION
Researchers have attempted to investigate the mechanism of codeposition to understand the way particles are incorporated into metal matrix over the past decades. Many models have been developed which gives insight into the mechanism of codeposition of particles on the cathode. Most models that have been developed involve one or more of the following processes:
(i) migration of the charged particles to the cathode by electrophoresis,
(ii) adsorption of the particles on the surface of the electrode by van der Waals forces and
(iii) mechanical incorporation of the particles into the layer [24]. In 1972, Guglielmi developed a two-step adsorption model that incorporates both adsorption and electrophoresis to describe the mechanism of particle codeposition. In the first step, the particles are covered with metal ions which cause them to be weakly adsorbed on the cathode.
The second step includes the strong adsorption of the particles by Coulomb force which result in subsequent entrapment of the particles within the metal matrix [25]. The model has been accepted by many researchers; however, it excludes the particle characteristics and mass transfer during electrodeposition process. This model has been improved by other authors to eliminate the drawback associated with it. [26] used three modes of current density (low, intermediate and high) to differentiate the reduction of adsorbed ions on the particles.
The model was developed to improve Guglielmi’s model. It involves three steps, namely:
(i) forced convective-diffusion of particles to the surface of the cathode,
(ii) loose adsorption of particles on the cathode and
(iii) irreversible incorporation of particles by reduction of adsorbed ions. Bercot et al. [27] improved Guglielmi’s model by incorporating the corrective factor (third-order polynomial equation) to cater for its drawbacks. One model that has been greatly accepted by scholars is Kurozaki’s model, which includes the movement of the particles to the cathode by mechanical agitation. This model involves three-step processes for incorporation of solid particles in to a metal matrix, which include:
1. Uniformly distributed particles are conveyed to the electric double layer by mechanical agitation.
2. Charged particles are transported to the cathode surface by electrophoresis.
3. Particles are adsorbed at the surface of the cathode due to the Coulomb force between the particles and adsorbed anions.
Figure 4 demonstrates the five consecutive steps involved in codeposition of particles into metal matrixes. The regions include formation of ionic cloud around the particles, convective movement towards the cathode (convection layer, typical length less <1 mm), diffusion through a concentration boundary layer (diffusion layer, typical dimensions of hundreds of µm), electrical double layer (typical dimensions of nm) followed by adsorption and entrapment of particles [28]. This model shows the step by step progression of particle codeposition from the electrolytic bulk solution until they are incorporated into the metal matrix. An electro-active cloud that surrounds the particles that are added into the electrolyte is created, which transport the covered particles to the hydrodynamic boundary layer through convection.
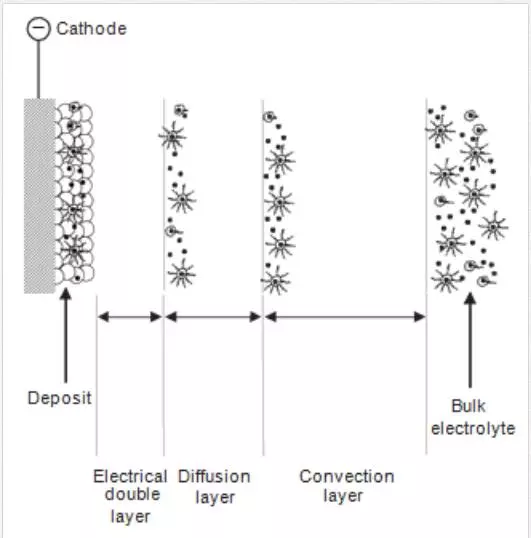

The particles migrate through this layer and are taken to the cathode through diffusion. The deposited and incorporated inert particles are embedded into the matrix by discharged metal ions after the ionic cloud is wholly or partially reduced [24]. The manner of incorporation of particles on the metal matrix depends mainly on the electrodeposition process parameters. Some of the most important parameters include the speed at which the bath is stirred, the applied current density and electrolyte composition. Bath agitation serve as a medium that assists particles to be transported to the cathode; while applied current density and electrolyte composition are responsible for the formation of ionic cloud around the introduced particles. There are three possibilities for particles to be incorporated into a metal matrix: (i) coatings that are just covered by adsorbed particles on the surface, (ii) coatings containing entrapped particles and (iii) coatings containing particles truly embedded uniformly into the metal matrix [29].
These three possibilities for explaining the manner of particle incorporation into the matrix is clearly demonstrated in Figure 5.
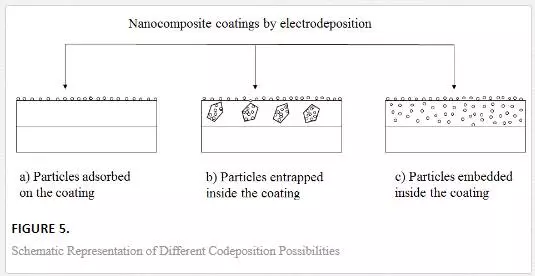
The first type of coating that comprises many adsorbed particles on the surface is shown in Figure 5(a). These type of coatings exhibit inferior chemical, mechanical and thermal stability properties due to the unoccupied crevices, pores and microholes on the surface of the coatings. The localized inclusion of particles in the metal matrix as shown in Figure 5(b) is caused by agglomeration of particles in the solution and rapid deposition rate occurring as an effect of unoptimized process parameters. The poor performance of these coatings due to inhomogenous distribution of particles that causes surface defects does not allow them to be used for technological applications. The third coatings with truly homogenous and embedded particles within the matrix are corrosion and wear resistant possessing excellent microhardness [29]
EFFECT OF OPERATING PARAMETERS
Incorporation of composite nanoparticles into metal coatings depends mainly on the experimental parameters. The amount and nature of incorporation of the particles in the metal matrix determine the quality and success of the fabrication of the composite coatings. Several common process parameters and their effect on the properties of the coatings are discussed below.
APPLIED CURRENT DENSITY
Current is one of the important experimental parameters that require optimization in electrodeposition since the success of the process depends solely on current. The process uses electrical current to accelerate the dissolution and flowing of electrons to be deposited at the cathode. Studies have shown that at lower current densities, harder and brighter coatings with increased concentration of incorporated particles are formed. Current density of 0.8 A/cm2 yielded microhardness value of 400 Hk, and increasing the current density to 1.6 A/cm2 resulted in a decrease of 340 Hk. The mechanism of codeposition can be explained by the fact that at lower current densities, more time for achieving certain thickness is required compared to higher current densities. Due to this requirement, availability of particles at the cathode is raised and the possibility of the particles to be uniformly embedded in the coatings is increased. This results in formation of good quality composite coatings that can withstand trying environments [30]. [31] reported no significant effect on the microstructure and uniform distribution of Al2O3 nanoparticles in the nickel matrix due to the variation of current density from 0.01 to 0.03 A/cm2. However, the concentration of the particles in the coatings was found to vary depending on the experimental condition (current density) the deposit was fabricated from. At low current density, increased concentrations of Al2O3 nanoparticles were present.[32] studied the influence of applied current on the microstructure of electrodeposited ceria coatings for enhanced oxidation resistance of nickel-based alloy. The results showed that high applied current density lead to formation of crack network on the microstructure of the coatings. The development of the crack network was attributed to the rising of internal stresses caused by rapid deposition kinetics [32]. Increased current densities enhance the deposition kinetics and promote rapid codeposition, which forms coatings that exhibit no improved properties. Increased voltage gives results that are in contrast with findings obtained with variation of applied current density. Weight gain, brightness, fineness and adhesion were improved when applied voltage was increased from 0.6 to 0.8 V [33] Other reported work showed that cathode current efficiency was achieved at low current densities when the influence of process parameters on cathode current efficiency were studied on electrodeposition of Zn–SiO2composite coatings [34].
PARTICLE CHARACTERISTICS AND CONCENTRATION IN THE BATH
The size, shape, type and concentration of the particles used for the fabrication of composite coatings have significant influence on the manner of incorporation mechanism of codepostion and properties of the subsequent coatings. The incorporation of metal oxide, carbides, nitrides, borides and so on has been reported to exhibit excellent surface properties by several authors [24, 35-37]. However, it has been found that micron- and submicron-sized particles incorporated into the coatings leave surface defects such as microholes, pores and crevices on the metal matrix, which then serve as a weak site for material degradation when exposed to a corrosive medium [38]. Findings have shown that when the size becomes smaller there are high chances for more of the particles to be codeposited into the matrix. Solution where 300 nm Al2O3 particles were used resulted in low amount of particles present in the nickel coatings compared to solutions where a size of 50 nm was used [38]. Nanoparticles possess excellent and exotic properties that are not contained in the bulk material even when they are of the same kind [15]. Incorporation of metal oxide, carbides and nitride nanoparticles yielded high microhardness, better corrosion resistance, good thermal stability and wear resistance [39, 36, 40].
The concentration of the particles in the electrolytic bath has significant effect on the properties of the coatings, and different particles reach optimum concentration in the solution at different bath loading levels. ZrO2 nanoparticles were found to yield best microhardness values when the bath loading was at 5 g/L during their incorporation into a zinc matrix. The results showed a linear relationship between the particle concentration in the bath and their contents in the coating [36]. This behaviour is in harmony with Guglielmi’s two-step adsorption model. A linear relationship between current efficiency and bath particle loading was observed by [34]. The increasing concentration of silica particles in the bath resulted in reduction of the current efficiency. The decrease might have been caused by solution resistance due to the increasing concentration of SiO2 particles in the solution. This contrasting result suggests that every particle has its own peculiar behaviour when added into bath solution and hence unpredictable. The work reported by [41] showed that the optimum particle loading for Al2O3incorporated into Zn–Ni matrix is 5 g/L and deviation from this value resulted in poor electrochemical behaviour of the coatings. The reduced corrosion inhibition of the particles beyond the optimal concentration has been ascribed to agglomeration of the particles in the bath, leading to lack of availability of particles to be deposited on the cathode. Another reason is the development of poor quality coatings caused by the generation of defects in the coatings due to rapid codeposition. Nano-Cr2O3 was effective at 50 g/L and increment to 75 g/L resulted in no significant effect on the microhardness of nickel-based coatings [30]. Good anti-corrosive properties, better thermal stability and microhardness have been achieved by adjustment of bath particle of SiO2 to 5 g/L in a Zn–Ni matrix.
BATH TEMPERATURE
Temperature does not play a major role in electrodeposition as it does in electroless plating, which depends mainly on the temperature and pH of the bath. However, increasing of temperature is known to enhance reaction rates in chemical processes. High temperatures increase the supply of ions to the cathode and the rate of nucleation of particles at the cathode [42]. This behaviour of the particle due to increases in the temperature has negative impact on the quality of the coatings. [12] reported the increase of the content of cobalt in zinc coatings due to the increment of temperature. However, raising the temperature was also found to reduce the polarization potentials and increase the amount of zinc in the deposit. So it is important for optimum temperature to be established to avoid all the drawbacks that are associated with increases in deposition temperature.
BATH AGITATION
The agitation of the bath during electrodeposition serves two functions: keeping the nanoparticles in suspension and the transportation of the particles to the cathode for codeposition. This actually explains that the incorporation of the particles in the metal matrix depends on the agitation speed of the bath during electrodeposition. Increasing of agitation speed improves the chance of the availability and adsorption of the particles by entrapment into the matrix. However, speeds beyond the optimal levels remove the particles from the cathode before they can be incorporated. The study of the effect of agitation speed on the microhardness revealed an increase in microhardness to maximum value of 395 Hk at 400 rev/min. Further increase to 600 rev/min had no effect on the microhardness of the coatings [30]. Agitation speeds of 900 and 1200 rev/min gave good microstructures when SiC particles were incorporated into a Ni matrix. Deviation from these values resulted in undesired appearance of microstructure, and it was concluded that the agitation must neither be too low or high but optimum [43].Figure 6 demonstrates the effect of rotation speed on the incorporation of polytetrafluoroethylene (PTFE) particles in nickel coatings as studied by [27]. Different optimum rotation speeds were established, depending on the particle loading which is 500 rev/min at 10 g/dm3, 600 rev/min at 20 g/dm3 and 700 rev/min at 30 g/dm3. Similar results were obtained when two baths were investigated for the effect of agitation speed on the current efficiency. The bath that contained 26 g/L SiO2 showed higher current efficiency compared with the bath loaded with 13 g/L. The increase in current efficiency was attributed to enhanced mass transfer due to agitation action [34]. These results showed that achieving optimum rotation speed depends on the particle concentration of the solution.
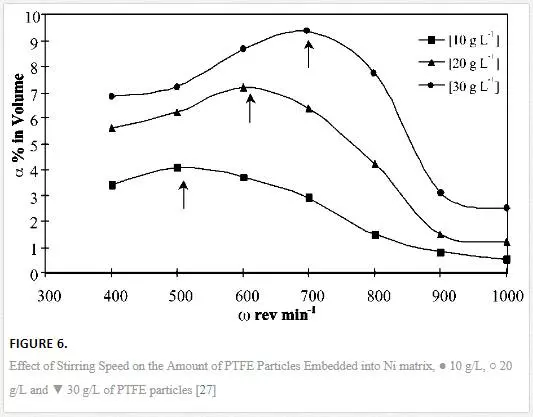
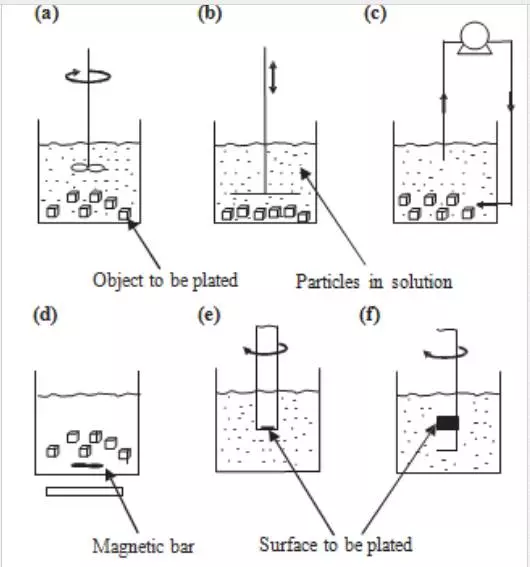
Figure 7 shows methods that are used in industry and laboratories to keep particles in suspension and transport them to the cathode during electroplating. These include physical dispersion of particles by bath agitation (mechanical stirrers, reciprocating plates, pumping, rotating disk or cylinder electrodes and parallel plate channel flow) and chemical dispersion by surfactants (cationic surfactants or combination of cationic and anionic surfactants).
ELECTROLYTIC PH
Bath electrolytic pH has an influence on the behaviour of the bath since reactions can only occur under certain pH conditions. [45] reported an increase in deposition rate due to increasing electrolyte pH in electroless plating of Ni–Fe–P alloys. However, pH values higher than 9 was found to cause solution instability due to precipitation of ferrous hydroxide during plating. Hypophosphite oxidation influences the rate of deposition, and this reaction is dependent on the pH of the solution. Electrokinetic measurements showed ZrO2 nanoparticles were positively charged in pH less than 4 which aided the particles to be transported to the cathode. The addition of the particles in solutions of pH 3.5 and 2.5 did not cause any new process, but some kind of surface blockage occurred on the electrode favouring reduction reactions [36]. The reduction reactions that could possibly occur are shown in Equations 11 and 12 below:
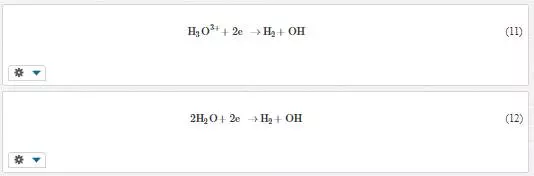
The formation of inhibiting species such as hydrogen and nickel hydroxide depends on the pH of the bath and other process parameters. Hydroxide inhibiting species forms at high pH, and the evolution of hydrogen occurs at low pH as depicted by Equations 5 and 6. The occurrence of this species affects the degree of texture of the coatings, and it can be easily controlled by adjusting the pH. The preferential orientation of Ni–Cu films of (100) at pH between 3.3 and 2.6 was changed to (111) by changing the electrolytic pH to 2 [46]. [47] obtained insufficient zinc coating in the low current density region at low pH between 2.5 and 3.5. The increasing of the pH to 4 yielded satisfactory results with a bright and mirror finish deposit. Further increasing of the pH above 4.5 gave negative results and the deposit became dull. The activity of bath additives depends also on the electrolytic pH, and hence it is important to optimize the pH when best results are sought.
DEPOSITION TIME
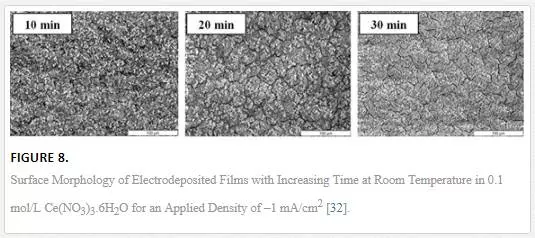
The thickness and the amount of incorporated nanoparticles in the composite coatings depend on the deposition time. [34] studied the effect of deposition time on the current efficiency of electrodeposited Zn–SiO2 coatings. The current efficiency was found to increase with deposition time; the optimum time established was 180 seconds. Beyond this point, the current efficiency started to reduce which might be due to agglomeration of the particles in the bath. When these agglomerated particles are codeposited, blocking on the cathode due to the reduction of active surface area for deposition and a subsequent decrease in current efficiency occur [34]. Three distinct regions have been observed on the coating: a relatively compact underlayer of zinc, a discontinuous middle layer of dendritic trees of zinc with vertical silica in between and a topcoat of predominantly dense silica. The development and build up of these layers occur as a function of pH and time. The first layer of zinc forms with the good current efficiency due to the availability of the active surface area. The thickness of the coatings increases with time and pH also increases, causing agglomeration of particles in solution. The codeposition of the agglomerated particles reduce the active surface area and localized deposition, leading to the development of zinc dendritic ‘trees’ struggling to build up through the thick mass of silica top coat. The increase in the thickness of the coatings depends not only on the time but also on the bath particle loading. [48] obtained similar results when investigating the effect of time on the weight gain of zinc deposits.[32] studied the influence of deposition time on the deposit growth, current density and coating thickness. Figure 8 shows the relationship between deposition time, deposit weight gain, thickness and the microstructures obtained from different deposition times. The results showed that deposit growth and thickness was not affected by the change in current density but increased with electrodeposition time. The deposit weight gain and thickness was constant up to 30 minutes of plating process. However, the coatings produced beyond 10 minutes of electrodeposition revealed a growing number of cracks [32].
CHEMICAL ADDITIVES
Chemical additives play a major role in electrodeposition. These chemical agents (surfactants and stabilizers) enhance the brightness, induce grain refinement and improve deposit properties. The commonly used additives for electrodeposition of zinc include gelatin, dextrin, thiourea, glycin and glycerol [49]. Others that have been recently reported include CTAB (Cetyltriammonium Bromide), SDS (Sodium Dodecyl Sulphate), TEA and MEA [4, 36, 50]. Their addition during plating has a significant effect on the resultant deposits. They alter crystallographic orientation of the matrix and perturb grain growth, leading to microstructures with small-sized grains. High concentrations of the surfactant in the bath have been reported to positively affect the microstructural properties of the produced deposits [49]. The added additives also increased the content of the reinforcement particles and also control their distribution in the matrix.
Properties of electrodeposited composite coatings
Zinc matrix nanocomposite coatings find various applications in industry due to the unique properties that they exhibit over the traditional metal or alloy coatings. There are several composite coatings that have been produced using electrodeposition, but the ones that have received most commercial use include zinc and zinc-nickel nanocomposites. These novel coatings have been developed to meet the industrial demand and perform better under demanding environments at a reduced thickness than traditional coatings [29]. Zinc and zinc-nickel composite coatings exhibit enhanced properties such as self-healing properties, high hardness, good thermal stability, corrosion and wear resistance.
ZN MATRIX COMPOSITES
Electrodeposition of zinc composite coatings is motivated by the development of novel coatings with enhanced surface properties for combating corrosion phenomena. Due to the ability of Zn to sacrificially protect steel from corrosion, it makes the metal to be an attractive matrix for fabrication of advanced composite coatings for demanding application. Several inert second phase particulates have been incorporated into the matrix, and improvement in the surface characteristics of the base coatings was achieved. The choice of the particles to be incorporated and formulation are guided by the properties expected to be achieved and potential applications where the coatings can be used. Single or double particle loading to fabricate binary and ternary composite coatings has been reported in literature.
COMPOSITION, MORPHOLOGICAL AND STRUCTURAL PROPERTIES
The incorporation of 5 and 10 g/L of sub-micron Al2O3 into Zn matrix were found to follow the morphological orientation of Zn coating but with partial homogeneous structures [24]. The particles were also found to have no chemical interaction with the matrix, and the particle content in the coating was subject to the concentration of the particle in the plating bath. However, [51] reported formation of ZnxSnx phases when SnO2 was codeposited with Zn. The chemical interaction observed in this work may be due to the change in operating parameters and bath formulations. The solubility of particles depends on various factors, and temperature and pH play a major role. Therefore, the difference obtained by the authors may be due to the increment of operating temperature to 40 °C. The addition of 7 g/L of the reinforcement particles yielded microstructures with no surface pores. The grain size was also found to be not dependent on the content of particles in the bath. [49] observed a correlation in bath particle loading and content of incorporated particles in the coating. Increasing particle concentration in the electrolyte solution improved the codeposition of the particles (bath solutions with high particle concentration yielded coatings with high particle content). This result is supported by Guglielmi’s two-step adsorption model [25]. The model suggests that increasing the particle content in the bath increases their availability to the cathode thus enhancing codeposition.
CORROSION RESISTANCE
The main challenge that is faced by steels in marine and coastal environments is corrosion degradation, and enhanced corrosion resistance is one of the key properties that the fabricated coatings must exhibit. The chloride in the sea water accelerates material loss in this atmosphere. Zn–TiO2 composite coatings exposed corrosive atmosphere of 3.65% NaCl proved to possess better corrosion resistance than mild steel as studied by [35]. The embodiment of the particles brought a positive potential shift of 0.38 V and decreased the current density from 0.07 to 0.0000211 A/cm2. Similar results were obtained by the authors when SnO2 particles were incorporated into the matrix. The inclusion of the second phase particles into the metallic matrix induces formation of stable film for further protection against corrosion. The passive film acts a physical barrier separating the fresh matrix from the corrosive media. The distribution of particles in the matrix also has a strong bearing on the corrosion resistance. Samples with uniform distributed particles have better anti-corrosive properties than those with agglomerated particles. Therefore, it is mandatory for the production of the composite coatings to be optimized to produce coatings with better particle dispersion and enhanced microstructure.
OTHER PROPERTIES
HARDNESS
Incorporation of submicron particles into metal matrix is known mainly for their excellent improvement of mechanical properties. The fabrication of Zn composite coatings from baths solution containing 5 and 10 g/L of submicron alumina particles drastically increased the hardness of Zn from 1.77 to 2.55 and 2.37 GPa, respectively [24]. The irregular morphological characteristics exhibited by samples fabricated from 10 g/L might have been responsible for reduction in hardness. Incorporation of second phase particles into the matrix impedes the growth of grains leading to the formation of microstructures with small-sized crystals [36]. Therefore, uneven distribution of particles in the matrix promotes formation of microstructures with chemical and physical inhomogeneity. Multiple loading of particles has also been found to improve the matrix by [52]. The incorporation of TiC–TiB mixed ceramic particles into Zn matrix have positive synergetic effect on the mechanical properties. The ternary composite exhibited high hardness values as compared to Zn–TiC and Zn–TiB. The synergetic properties of the mixed reinforcement particles improve the properties of the individual ones.
WEAR RESISTANCE
Wear resistance is one of extractive properties that coatings should possess for durability and increased life span during service. However, zinc coatings are known for their poor performance when they are subject to wear environment. The development of composite coatings also aims at improving this property to extend the applications of the coatings. The inclusion of reinforcement particles hinder plastic deformation due to the dispersive strengthening effect exhibited by the particles [51]. The authors also reported that the alteration of microstructure and strengthening of the coatings by formation of compact and crack-free coating add to the minimization of friction due to the presence of embedded particles The particles also form a protective barrier between the matrix and the wearing medium, thus reducing direct contact and friction coefficient [53].
ZN NANOCOMPOSITE COATINGS
MORPHOLOGICAL AND STRUCTURAL PROPERTIES
The inclusion of nanoparticles in a metal matrix has been reported to induce grain refinement and modify the structural characteristics of the matrix. During codeposition, second phase particles are adsorbed on the growing crystal and retard its further growth. The adsorbed particles increase the number of nucleation sites, which results in reduction of the grain size [36]. The morphology of composite coatings depends mainly on the manner of particle dispersion and content of particles in the metal matrix [4]. Morphological and structural analysis results obtained by [54] revealed a reduction of crystal size of Zn matrix due to the incorporation of TiO2 nanoparticles. The nanocomposite coating followed the same morphological pattern with the matrix, but with smaller and smooth grains. The deposits also showed minimal surface defects such as pores and microholes. Zn–TiO2 nanocomposites produced from baths containing the highest particle concentration (16 g/L) have been reported to affect the morphology of the plain Zn coatings [37]. The nanocomposite coatings exhibited surface morphology different from the matrix. High particle content and orientation of particles (agglomeration) in the coating causes an evolution of a new structure. The introduction of SiC were found to cause deposition overpotential leading to the formation of coatings with grains perpendicularly oriented to the substrate as compared to the parallel hexagonal plates showed by Zn matrix [55]. The incorporation of the particles onto the cathode was also reported to perturb horizontal grain growth in the coatings. Therefore, the morphological characteristics of the coatings depend solely on the bath parameters and formulation.
CORROSION RESISTANCE
Addition of second phase particles to Zn matrix promotes the formation of fine microstructure with good corrosion resistance. The particles perturb the growth of Zn crystals and induce the formation of new nucleation sites resulting in small-sized crystals and a microstructure with minimal defects [13]. Uniform dispersion of the nanoparticulates in the matrix fills the microholes, pores, crevices, gaps and other surface defects of the coatings leading to the formation of compact microstructures. These defects can serve as active sites for corrosion if present. The particles have also been reported to act as physical barriers between corrosive media and the matrix, thus minimizing the occurrence of defect corrosion [55]. Codeposition of black carbon nano-sized particles with Zn reduced the current density when the coatings were tested in 3.5% sodium chloride solution [56]. The metal dissolution of the composites was also found to occur at high anodic potentials at a steady rate as compared to the Zn coating. [36] reported zirconia nanoparticles to influence the kinetics of the anodic and cathodic electrochemical reactions. Therefore, the particles can promote the perturbation of anodic reaction and favour the formation of a stable passivation layer. Therefore, the presence of second phase particles in the matrix has positive effect on the corrosion resistance of the coatings. However, optimum particle loading has to be established to obtain coatings with enhanced anti-corrosive properties. Agglomeration of particles in the coating was found to occur when Al2O3 and SiO2 particle contents in the electrolyte solution were beyond optimal concentrations [57]. The authors established optimal particle loading for excellent corrosion resistance is 5 g/L for both Al2O3 and SiO2. Beyond these optimal conditions, the corrosion resistance of the coatings was compromised. Incorporation of non-uniform agglomerates in the matrix promotes formation of surface defects and causes chemical heterogeneities in the coating. This allows penetration of the corrosive media into the matrix in weak sites, leading to the degradation of the corrosion resistance of the entire system.
OTHER PROPERTIES
HARDNESS
Microhardness of a metal matrix mainly depends on the microstructural properties of that particular matrix. When force or load is exerted on a material, the dislocation movements are induced which lead to the weakening of the material’s mechanical properties [58]. Incorporation of nanoparticles into a matrix perturbs dislocation movement and grain boundary sliding, thus improving the hardness of the composite coatings. Grain refinement and uniform incorporation of reinforced particles result in improvement of hardness. [56] obtained an increase in microhardness from 72 to 90 HV when 2 g/L of nano-sized carbon particles were incorporated into Zn matrix. The composite coating also showed improved morphology with small-sized crystals. Another factor that affects microhardness of a Zn matrix is the content of the reinforcement material added into the coating. The increase of bath particle content increases the chances of the particles to be incorporated into the metal matrix [54-55].
WEAR RESISTANCE
The coatings’ resistance to wear degradation is one of the important properties sought for metal matrix composites to exhibit. Many industrial applications where these coatings are employed require them to possess excellent wear resistance. The addition of reinforcement into a metal matrix promotes grain reduction and hinders plastic deformation of the material [59]. This is attributed to particles’ grain refining and dispersive strengthening characteristics exhibited by these additives. The reinforcement particles also possess self-lubricating properties and hence reduce friction, thus improving the wear resistance of the matrix. The incorporation of nano-sized TiO2 into Zn matrix carried out by [54] reduced the wear loss of Zn from 11 to 5 mg. The results obtained by [35] showed an improvement in wear resistance and reduction of friction coefficient of the Zn matrix due to the addition of TiO2particles.
ZN ALLOY NANOCOMPOSITE COATINGS
There are several zinc alloy nanocomposite coatings ranging from Zn-Ni, Zn-Co, Zn-Fe, etc. to matrix-based composites. Zn-Ni matrix nanocomposites have received much attention as compared to the others. Nickel is chemically stable and has better mechanical properties than Zn, and its incorporation into Zn matrix notably improves the corrosion and wear resistance of the coatings.
COMPOSITION, MORPHOLOGICAL AND STRUCTURAL PROPERTIES
Uniformly distributed nano-sized Al2O3 incorporated into Zn-Ni matrix improves the compaction of grains in the coatings and minimizes surface defects [60]. The crystallite size of Zn-Ni matrix was reduced from 40.9 to 20.68 nm. The crystallite size was found to be dependent on the particle content of nano-Al2O3 present in the solution. The authors also reported the increment of nickel content in the coatings due to the addition of the reinforcement particles. The work conducted by [41] confirms these findings. Zn5Ni21 phase was increased as the result of incorporation of the nanoparticles into the matrix. [39] also showed similar results when SiO2 nanoparticles were added. The nickel content was increased from 6.3 to 12.3 wt.% while zinc was decreased from 93.7 to 87.7 wt.%. Inclusion of particles into the matrix has significant influence on the surface morphology of the resulted deposits. Al2O3 solutions changed hemispherical nodules of Zn-Ni to cauliflower-like surface morphology [61].
CORROSION RESISTANCE
The automobile industry extensively uses Zn-Ni coatings for corrosion protection of steel. These coatings are chemically stable than their zinc counterparts. Addition of second phase nanoparticles into Zn-Ni enhances anti-corrosive and mechanical properties. Al2O3 nanoparticles have been incorporated into Zn-Ni matrix from alkaline solutions by [41] The coatings fabricated from baths containing 5g/L Al2O3 possessed excellent corrosion resistance when tested in 0.2g/L Na2SO4 electrolyte. The corrosion current was drastically reduced, and the polarization resistance increased. These findings are similar to those obtained by [60] where the current density was decreased from 0.19 to 0.092 µA/cm2. The results obtained by the authors show that the particles impart inhibitory effect to the corrosion of the matrix. Al2O3 particles exhibit low level of electronic conductivity and can disturb corrosion current when they present in the coating. SiO2 nanoparticles codeposited with Zn-Ni matrix have reported to protect the matrix from corrosion in 3% NaCl solution [39]. Electronegative Zn rich η phase has been found to improve the protective life of the coatings.
OTHER PROPERTIES
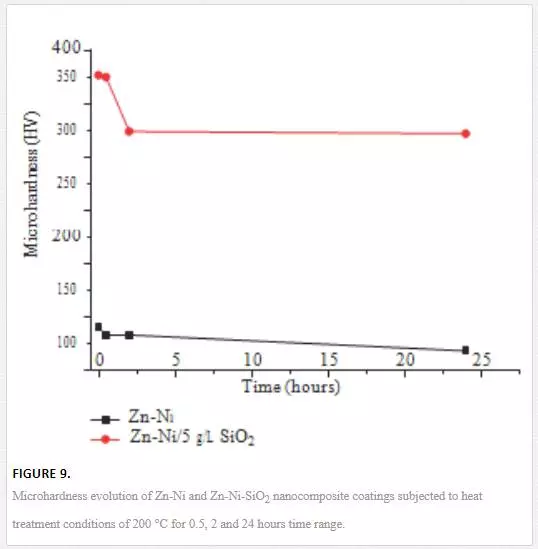
Other properties that Zn-Ni–based nanocomposite coatings possess include high hardness and good thermal stability. [60] obtained improvement of microhardness of 640 HV when they incorporated Al2O3 nanoparticles into Zn-Ni matrix with the aid of ultrasound for bath agitation. Zn–Ni–SiO2nanocomposite coatings produced from bath containing 5g/L SiO2 increased the microhardness value of the matrix from 140 to 396 HV [39]. The microhardness values of the composite remained higher than that of the matrix even when the samples were subjected to annealing temperatures of 200 °C for a duration of 24 hours (shown in Figure 9) Second phase reinforcement particles have pinning effect on the grain growth of the coatings due to elevated temperatures. The particles reduce surface oxidation and relaxation of internal stressed.
Conclusions
From the current review, it can be concluded that electrodeposited Zn composite coatings have high potential to serve as protective films for protecting components and pieces that are made of mild steel from corrosion in coastal and marine environments. Their improved surface properties such as corrosion resistance, high hardness, low friction and wear resistance due to the incorporation of second phase particles enable them to find various applications. The advantage of inclusion of inert ceramic particles in metal deposits is that harder, and chemically stable coatings can be produced at reduced thickness. However, the quality of electrodeposited coatings is not dependent on the presence of reinforcement particles only but on a number of process parameters (pH, plating current, temperature, deposition time, stirring speed and bath composition); hence, optimization of the process is required to produce desired and high quality coatings. More work on further improvement of electrodeposited zinc coatings is required to enable them to serve as alternative or replacements to other coatings produced by high cost and energy intensive surface modification processes.