Review of Mercury Circulation Changes in the Coastal Zone of Southern Baltic Sea
One of the coastal pollution sources is an oil spill, such as the Gulf of Mexico disaster [1]. However, coastal land use has a large impact on the quality of seawater. One coastal chemical pollution is mercury (Hg). Despite its undoubted usability, mercury (Hg) is the most toxic metal and one of the most toxic elements. It is as yet unknown whether there are any beneficial functions performed by mercury in live organisms. It is, however, proven to have neurotoxic, nephrotoxic, immunotoxic, mutagenic, embryotoxic, and allergising properties [2, 3]. It is one of the factors causing a number of disorders, such as Alzheimer’s disease, Parkinson’s disease, autism, amyotrophic lateral sclerosis, or lupus [2, 4–7]. The main route of mercury introduction to the human system is the consumption of fish and seafood. Owing to the fact that mercury is capable of penetrating quickly through the placenta (foetus barrier), some countries have introduced recommendations against the consumption of predatory fish by pregnant women [3].
The problem of mercury toxicity was only widely explored in the second half of the 20th century, following cases of fatal poisonings as a result of the consumption of contaminated fish [8] and grains preserved with mercury compounds [9]. As a consequence, Hg emission and deposition has been monitored in many regions of the world. In the Baltic region, the role has been performed by the Baltic Marine Environment Protection Commission, also known as the Helsinki Commission or HELCOM. The important pathway of Hg transport is the atmosphere, but in coastal zones, rivers represent the main source of the metal. According to HELCOM reports [10, 11], Hg emission in the Baltic region at the beginning of the 21st century was about 20–30% lower than during the 1980s. Climate warming, particularly in the autumn-winter season [12], is another factor contributing to the decline in Hg emission from the main source, the burning of fossil fuels, into the coastal zone of the southern Baltic. On the other hand, in the seaside air rich in halides, there are conducive conditions for a quick transformation of gaseous mercury to the aerosol form, which causes Hg to be removed faster to the ground. In the zone where marine and terrestrial air masses meet, this process is aided by high humidity. This results in larger aggregates being formed, which drop out of the atmosphere faster [13–15]. During warm autumn and winter months phytoplankton blooms can occur [16], leading to the bioaccumulation of mercury from atmospheric deposition, the inflow of which is always higher in the heating season as opposed to the warm part of the year [17]. In such cases, mercury compounds do not drop to the bottom of the water basin but are accumulated by phytoplankton and thus included in the trophic chain [18]. Climate changes in the coastal zone of the southern Baltic point to the shortening of the icing period [19], which affects the circulation of mercury between near-bottom water, sediments, and benthic organisms [20]. The reason for this is the prolongation of the macrophytobenthic vegetative period and zoobenthic activity, which can, in turn, increase the absorption of pollutants from near-bottom water and porewater and intensify the mixing of surface sediments. In recent years, the intensification of extreme natural phenomena has also been observed [12]. More frequent precipitation and floods can contribute to the reemission and remobilisation of mercury transported into the sea from land. The above observations led to the formulation of the following research goals: (I) to determine the factors that influence mercury concentration in aerosols, precipitation, and rivers in the southern Baltic region; (II) to determine the factors that influence the degree of Hg inflow via atmospheric precipitation and rivers into the southern Baltic; (III) to assess the role of macrophytobenthos as a carrier of mercury, transported to the sea contemporarily as well as in the past, into the trophic chain; and (IV) to determine the tendencies in the changes of Hg circulation in the marine environment under the influence of climate changes occurring in the southern Baltic region.
Materials and methods
The research was conducted at the coastal zone of Gulf of Gdańsk (southern Baltic Sea) (Figure 1) during 2005-2013. The solid samples were analysed by means of an advanced mercury analyser AMA 254 thermal desorption analyser. The detection limit for solid materials was 0.005 ngg-1.Water samples for mercury analysis were oxidised by the addition of BrCl and pre-reduced with hydroxylamine hydrochloride solution 1 h prior to analysis by CVAFS (TEKRAN 2600), according to US EPA method 1631 [21]. Quality control procedures for water samples included blanks and water spiked with mercury nitrate in the range of 0.5–25 ng/L and produced adequate precision (1% RSD) and recovery (98%–99%). The detection limit was as low as 0.05 ng/L. More details about sampling stations, collections, and chemical analysis are in publications [22–28].
Results
The research carried out during 2005–2013 has shown that, in the Polish coastal zone, mercury concentrations both in aerosols and in rains [23, 24] were comparable to the values obtained at other stations within the Baltic [29, 30, 32, 33]. The mean annual Hg concentration in aerosols (Hgp) (Figure 2) was 1% of total Hg concentration in the air, which is typical of unpolluted areas [22, 31].
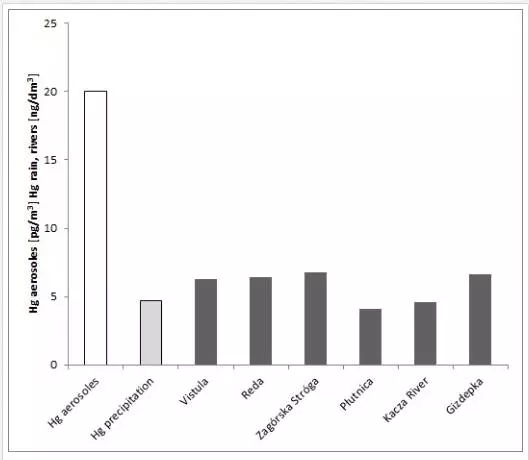
In the six studied rivers (Vistula, Reda, Zagórska Struga, Płutnica, Kacza River, and Gizdepka) terminating in the Baltic in the region of the Polish coast, the mean annual Hg concentrations were similar to the value of 5 ng Hg dm-3, which is considered to be the global mean concentration in watercourses. Annual medians were lower than 7 ng Hg dm-3 (Figure 2) [24, 25].
Discussion
CHANGES OF HG CONCENTRATION IN THE ATMOSPHERE AND RIVERS OF THE SOUTHERN BALTIC REGION
Hg concentrations in the air and rivers varied in the particular months of the year [22–25, 28]. In the region of the Polish coastal zone of the Baltic, at the station in Gdynia, the Hg concentration in large aerosols was higher than in small particles (on average, 93% of the total Hg in aerosols) [22]. The reverse was observed in air masses that had been transported from distant polar and subpolar regions high above the ground, particularly from over volcanoes in Iceland. A significant source of Hg in the southern Baltic region is the combustion of fossil fuels. Therefore, Hg concentrations in aerosols, both large and small, were higher in terms of statistical significance in the heating season than in the non-heating season (Figure 3). Moreover, in the heating season, Hg concentration in both large and small aerosols was lower in the maritime air masses than in continental air masses (Figure 3). This is related to the intensive combustion of fossil fuels for heating purposes in this season. The research showed a significant role of individual home furnaces, which are relatively numerous in and around the Tri-city, in the formation of mercury concentrations in aerosols and rains [22, 23]. Hg concentration in rains in both seasons (heating and non-heating) was not found to be different in terms of statistical significance [23]. In the coastal zone of the sea, a place where the polluted continental air masses meet the cleaner, humid and halogen-rich marine air masses, an important factor in forming the volume of mercury concentration, both in aerosols and in rains, was found to be that of Hg transformation. In such conditions, gaseous mercury Hg(0) can be oxidised to Hg(II) and adsorbed on condensation nuclei, which, in consequence, can lead to an increase of Hg concentration in rains and in large aerosols in marine sea masses, particularly in the warm season [22, 23]. These are significant processes, particularly in the warm season, when an increased emission of gaseous mercury from the sea into the air is observed [34]. As a consequence, in the non-heating season, Hg concentration in large aerosols was higher than in the maritime air masses compared to continental air masses [22], and Hg concentration in rains was the highest in summer (Figure 3) [23]. Additionally, in summer, mercury in dry air evaporated off the aerosol surface, which led to a drop in Hg(p) concentration in terrestrial air masses [22].
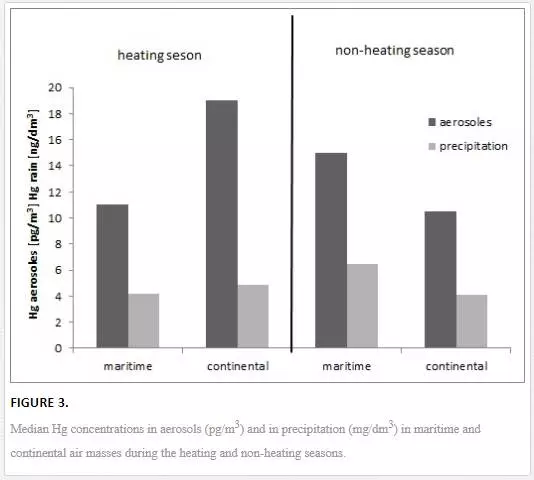
In rivers flowing into the Baltic along the Polish coast, Hg concentration was much less directly dependent on meteorological parameters than was the case with aerosols and rains. The level of mercury concentration in rivers was indirectly related to the volume and height of precipitation [24]. Depending on the type of catchment and the length and height of wet precipitation, Hg concentration was either diluted in a river or increased as a result of the metal being washed off land. An increase in mercury concentration was observed in sections of rivers flowing through urbanised areas. The washing off of pollutants from street and pavement surfaces, pitches, or car parks as a Hg source into rivers is also confirmed by increased metal concentrations in water from storm sewers collected both during and after rain. Hg remobilisation from agricultural areas also increased Hg concentration in rivers compared to the source section. In the 20th century, mercury compounds were commonly used in agriculture as fungicides, which is why such areas are currently a potential source of this metal into rivers and the sea [24].
FACTORS INFLUENCING HG INFLOW INTO THE SOUTHERN BALTIC
The inflow of mercury into the southern Baltic changed over the course of a year [25]. The size of Hg load introduced with dry precipitation mainly depended on air temperature, particularly in the heating season. In that period, dry deposition of Hg depended mainly on Hg concentration in aerosols in both marine and terrestrial air masses. Wet Hg deposition, on the other hand, increased with the height of precipitation. In the non-heating season, the size of Hg load introduced in this way depended on both the height of precipitation and the Hg concentration in rains and, analogously, that introduced with dry precipitation: on the concentration of Hg in aerosols and the deposition velocity [25].
Taking into account the average wind speed at the sampling station and the trajectories of air masses, the areas were defined from which mercury was brought to coastal zone: Vw < 1 m s-1 local sources; 1 ≤ Vw < 3 m s-1 regional sources; and Vw ≥ m s-1 remote sources [25]. There is a possible tendency of increasing Hg inflow from the atmosphere, which may reveal itself in the following conditions: in the heating season, the inflow of atmospheric Hg will increase with the number of cases where continental air masses from regional and distant sources, with the predominance of coal burning in home furnaces, flow over the coastal zone of the southern Baltic and with the increasing incidence of rains from marine air masses from distant sources (Figure 4) [25].
The atmospheric Hg load will also increase as air temperature drops and the amount of burnt coal increases, as well as with a rise in the intensity of gaseous mercury transformation into the particulate form [22, 25]. Taking into account the non-heating season, it was estimated that the more frequent are the inflows of air masses from regional sources (both marine and terrestrial) and rains from continental regional air masses, the greater the load of mercury coming from the atmosphere will be (Figure 4) [23, 25].
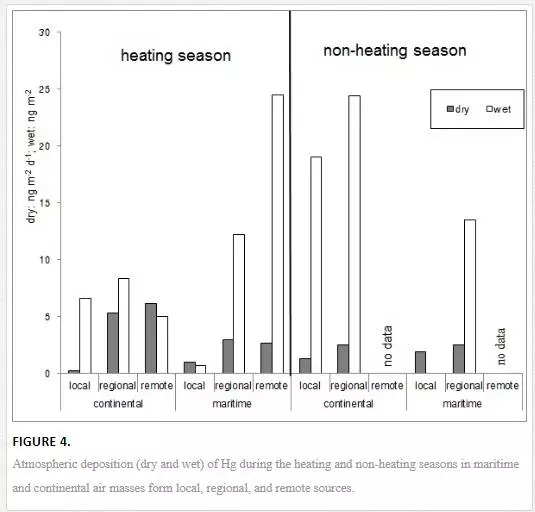
Hg inflow via rivers also fluctuated in the particular months over the course of the study period [25]. A larger load of mercury was introduced in the cold season, when the metal penetrated into rivers directly from increased atmospheric deposition and with the washing out of pollutants from land — those deposited there both contemporarily and in the past. In addition, meltwater also contributed to the transportation of Hg from land into rivers and the sea. Rains were also a significant factor forming the inflow of Hg via rivers. Changes occurred in various directions depending on the type of catchment: intensive rains were either conducive to the washing out of Hg from land or contributed to the thinning down of the metal concentration in the river.
MACROPHYTOBENTHOS AS A CARRIER OF HG INTO THE TROPHIC CHAIN IN THE COASTAL ZONE OF THE SOUTHERN BALTIC
Mercury that reaches the sea is either included in the trophic chain through accumulation by phytoplankton or phytobenthos or becomes sedimented in the seabed, from where it can be reintroduced to the cycle. A good indicator of the size and changes of metal concentrations in water is macrophytobenthos [35, 36]. It follows from the research on Hg concentration in macrophytobenthos, which has been carried out since 2006, that over a long time period the changes in concentrations in the particular seasons are statistically insignificant [26]. This is related to the disappearance of stark differences in air and water temperatures between particular seasons (the warming of the autumn-winter-spring season, lack of icing, and rainy summer). It has, however, been observed that there are differences in Hg concentration in macrophytobenthos in the particular regions of the Polish Baltic coast [26].
Benthic flora in the coastal zone of the open sea was considerably less polluted with mercury than the material from the Pomerania Bay or the Gulf of Gdansk. The urbanisation of the coastal zone has contributed to the increase in Hg concentration in macroalgae. Surface run-off and the inflow of rainwater (e.g. from storm sewers), to the coastal zone of the gulf, have led to an increase in mercury concentration in macroalgae there compared to regions located far away from those sources. On the other hand, the inflow of pollutants from river catchment areas contributed to an increase in the concentration of the metal in marine vascular plants. The suspension carried via rivers into the sea, depending on the type of the outlet, was deposited close to the shore to a smaller (e.g. Vistula outlet) or greater degree (e.g. Oder outlet), becoming an Hg source into porewater and, consequently, into vascular plants. The capability of vascular plants to accumulate chemical substances from porewater also contributed to the absorption of mercury that had been deposited in sediments in the past. As a consequence, Potamogeton pectinatus (a vascular plant) had a 60% higher bioconcentration coefficient than Furcellaria lumbricalis (a macroalga) [26].
POSSIBLE INFLUENCE OF CLIMATE CHANGES ON THE CIRCULATION OF HG IN THE COASTAL ZONE OF THE SOUTHERN BALTIC
In recent years, an intensification of extreme natural phenomena has been observed [12, 37]. Intensive rains and floods cause increased the washing off of chemical substances from land, which, in consequence, contributes to the inclusion in the river course of mercury deposited for decades. This is a highly significant problem, as calculations have shown that currently about 50% of mercury deposited into the bottom sediments of the Gdansk Basin becomes reintroduced to the water column. If more mercury flows into sediments (e.g. with surface run-off as a result of intensive rains), the streams of mercury emission from sediments may increase by a few to more than 10% above the difference resulting from the change in the inflow [38]. Thus, the bottom sediment will increase its share as a source of Hg into the marine ecosystem.
During the largest flood (2010) on the River Vistula, within a relatively short time (31 days), 1.2 tonnes of Hg penetrated into the Baltic, amounting to 75% of the annual load in 2010 [27]. Such a large mass of mercury introduced was caused not only by the large volume of water (12 km3, which amounted to 21% of the yearly flow of the Vistula) but also by a several-fold increase in the Hg concentration compared to the time before the flood (Figure 5). During the culmination of the first flood wave, the concentration of total mercury (Hg tot) in the Vistula was over 200 ng dm-3, whereas the Hg concentration median from before the flood was 6.3 ng dm-3. That was caused by the washing out of pollutants, deposited over years, from the soil, as well as from cemeteries, landfills, farmyards, waste depositories, etc. The high water level, persisting for a long time, apart from washing of Hg out of land, contributed to the deposition of suspension (enriched with mercury) close to and in the riverbed. This is of considerable significance as, under the influence of another rise in the water level, either due to a flood, flooding, or seasonal meltdown, it is very likely that mercury, in the form of methylmercury (the most toxic form), will be released from the bed and transported into the sea [27, 39, 40]. Such a situation was observed in July and September 2010 [25].
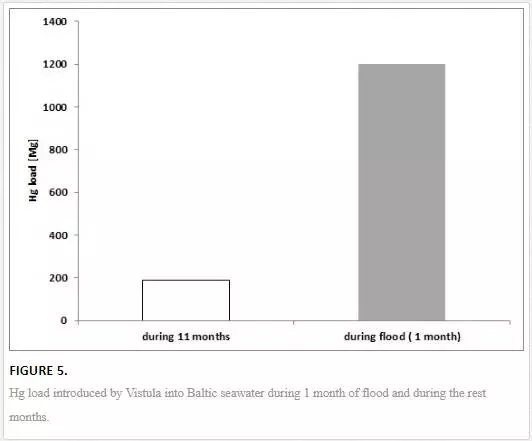
The mercury introduced into the coastal zone of the sea during the flood in 2010 was adsorbed by phytoplankton, contributing to a fourfold increase in its concentration in algae in the period between two flood waves reaching the Gdansk Basin, compared to the period before and after the flood [27]. Mercury bound to the fine sediment fraction, particulate suspended matter, and in its dissolved form was transported by the strong flood current from the river mouth far into the southern Baltic. As a consequence, the concentration of Hg in the sediments of the southern Baltic following the flood increased by as much as 500% compared to the period before the flood and, in relation to the fine fraction content, by as much as 1500%. This state persisted for nearly 2 years [27].
The mercury concentrations measured during the flood both in the Vistula and in the Gulf of Gdansk exceeded the safe values for water organisms. Hg concentrations in herring measured in 2011 by Woroń and Danowska [41] were the highest and were found to be 67% higher than in the period 1998–2010, while, in mussels, they were 60% higher than in that period [27].
According to HELCOM reports, mercury inflow into the Baltic has decreased since the 1990s, thanks to industrial modernisation [11]. Moreover, climate warming [12] has been contributing to the decrease in the proportion of the main Hg source in the Polish coastal zone (i.e. fossil fuel burning), which reduces the inflow of mercury with atmospheric deposition and rivers [25]. This is of particular importance in regions where emission from individual home furnaces is predominant. The inflow of Hg with dry deposition during winter, when subzero air temperatures were predominant, was eight times higher than in warm winter, abounding in temperatures above zero [25]. On the other hand, a warm winter, as opposed to a frosty one, is conducive to faster mercury transformation in the zone where the marine and terrestrial air masses meet, thus shortening the time of Hg residence and its transportation over long distances [22]. As a result of the lengthening of the warm season (winter starting later and ending earlier) [12], phytoplankton blooms are observed, particularly in the coastal zone, as early as the end of January or the beginning of February [16, 42, 43, 44]. In this situation, Hg from atmospheric deposition, the inflow of which by this route is always greater in the heating season than in the warm season [22, 25], does not drop to the bottom of the water basin but is accumulated by phytoplankton. As a consequence, Hg concentration in the phytoplankton of the Puck Bay was found to be higher in the heating season than in the non-heating season [28]. In this way, as a result of the warming of the winter season, taking into account the persistence of raised alga biomass, a higher Hg load is included in the trophic chain over the course of a year. During an anomal warm autumn (according to the rating introduced by the Institute of Meteorology and Water — the State Research Institute [37]), raised Hg concentrations were also observed in peryfitone covering stones [28]. Its mean concentration in that season was three times higher than during a thermally normal winter.
As a result of the warming of the winter season in the southern Baltic region, there are increasingly many winters with no icing even in the coastal zone of the gulfs, or with icing lasting much shorter [19], particularly compared to the years 1946–1991, when the average duration of the icing season in the inner Puck Bay was 90 days [45, 46]. The lack of icing is conducive to the thriving of macroalgae, which consequently prolongs the period of metal accumulation by fauna. On the one hand, the reduction of Hg emission into the environment has led to a drop in the concentration of the metal in macrophytobenthos of the Polish coastal zone of the southern Baltic [26]. On the other hand, the prolongation of the vegetative season and the improvement of environmental quality have led to an increasingly intense growth and coverage of the seabed by marine fauna. That has brought about the inclusion into the trophic chain of mercury deposited contemporarily as well as in the past. Seagrass beds are places where animal organisms thrive; they are also the consumers of phytobenthos, directly accumulating mercury. Thriving phytobenthos contributes to the faster inclusion of Hg into the trophic chain, and this is of particular importance in areas where the seagrass beds of Zostera marina are regrowing, as that is where the highest Hg concentrations were measured. In this way, while macrophytobenthos can be said to clear water and sediments, it also transfers Hg from sediments, which has relatively low bioaccessibility for higher organisms, to the higher trophic levels [26, 28]. High phytobenthos and zoobenthos biomass persisting during a warm autumn [37] caused the Hg mass contained in benthic organisms in that season to be five times higher than during a thermally normal winter [28, 37]. Owing to that, despite the decreasing Hg emission, the warming of the climate in the southern Baltic region is conducive to the prolongation of the period within which mercury is included in the food chain. Between 1999 and 2014, there were 12 instances in which autumn was observed to be above the thermal norm [37].
Climate changes are also observed in the summer. Increase of temperature led to enhanced emission/re-emission of gaseous Hg from seawater [34, 47]. In warm, marine air masses, there is intensive transformation of gaseous mercury into the particulate form, and rains occurring at that time lead to an increase in the Hg load introduced to the sea with wet precipitation [23]. In July and August, there is a higher incidence of intensive rains, reaching as much as 220% of the mean total precipitation from the period between 1971 and 2000 [37]. This leads to an increase of about 12% to the annual inflow of atmospheric Hg [28]. Intensive rains, particularly after a dry spell, were also favourable for the washing out of Hg from the catchment area, which also boosted the load of Hg introduced to the sea with rivers [24, 25]. This is of great significance, as at such a time phytoplankton biomass in the sea is normally high, presenting conditions conducive to the inclusion of a higher Hg load to the trophic chain, in comparison to a summer without anomalously intensive rains.
Conclusion
The presented phenomena and processes are especially significant for marine organisms that thrive in the coastal zone of bays. This is of particular importance, as these regions are attractive for tourists and consumers of fish, which are caught in these parts along with occasional seafood.Owing to the high toxicity of mercury, the Baltic countries are obliged to reduce its emission into the environment. The main source of Hg is the burning of fossil fuels, which is why an increase in its inflow to the Baltic may be observed in the heating season compared to the non-heating season. Many restrictions concern the energy sector. The conducted studies showed that not only industrial coal combustion but also regional individual home furnaces have an important influence on the deposition of this metal. However, along with the adopted methods of mercury removal from fumes, an important factor influencing the emission of this metal is the warming of the cold season: late autumn-winter, early spring. Anomalously high air temperatures translate to less heating needs, which in turn reduces coal burning. On the other hand, the warming of the winter season prolongs the vegetative season of marine organisms, extending the period within which Hg is included in the food chain. As a consequence, despite the decreasing mercury emission into the natural environment, a larger load of the metal may be included in the marine trophic chain over the course of a year.
The decrease in Hg inflow to the Baltic is not the only result of climate changes. More intense rainfalls also lead to the washing out of Hg from both the atmosphere and from land. The conducted studies showed that the remobilisation and discharge of Hg occurs in most catchments of rivers flowing into the Gulf of Gdansk. This, to a large extent, is the effect of urbanisation. Tarmac-ing or pouring concrete over roads and pavements limits the retention of Hg in the soil. Moreover, intensive rains and floods wash out the metal from agricultural areas where it was widely used in the past as a fungicide. This is why the type of catchment ought to be taken into consideration while drawing up regulations concerning the reduction of Hg emission by particular states. The present studies were conducted in the drainage area of the Gulf of Gdansk, but analogous processes occur in other regions on the same latitude. The choice of suitable methods of drainage area management may significantly regulate the discharge of Hg into the sea.Chemical substances that reached the sea with the extreme Vistula flood in 2010 were both deposited in the coastal zone and being carried far out into the Baltic, contributing to an increase in their concentrations in surface sediments. The research carried out after the flood indicated the need to monitor this process in sediments and, more importantly, in commercially attractive fish at least 2 years after the flood. Moreover, during a flood, a large load of polluted suspension drops to the riverbed or on the flooded areas, from where it can be washed off and transported to the sea during the next intensive floods or meltdowns.
The increase in rainfall, particularly in the summer, is of particular importance for the marine environment. This is related to an increased inflow of Hg with wet precipitation, but the warm season is also favourable for intensive growth of sea organisms and, consequently, a faster accumulation of chemical substances, including toxic ones. As a result, the concentration of mercury in organism biomass increases. The conclusions described above should be taken into account while preparing regulations concerning Hg emission by particular countries. Studies have shown that an increase in mercury concentration in marine organisms is not always linked to an increased inflow of anthropogenic Hg, directly influenced by humans, but may also be related to climate changes occurring in a given area, the type of catchment, as well as to the predominance of either marine or terrestrial air masses.The conducted studies show that even if Hg emission remains the same, its inflow into the Baltic will change as conditions, such as air temperature, number and intensity of rains, wind speed, and type of atmospheric circulation, change.