Stable Isotope Techniques to Address Coastal Marine Pollution
Marine pollution due to anthropogenic activities has now become a worldwide environmental concern [33]. Several researchers [9, 53, 60, W.Q.C., 1972;] have reported the influence of the indiscriminate discharge of untreated industrial effluent and municipal wastewater on the marine environment in terms of danger to habitats, serious risk to marine life, deterioration of aesthetic values, and limited access to coastal areas. Hence, monitoring of marine coastal environment is essential to understand the origin, distribution, fate, and behavior of marine pollutants to formulate a viable management strategy [17]. A variety of techniques ranging from conventional methods to most sophisticated isotopic techniques are available for monitoring the marine coastal pollution [15]. Isotope analysis is an engineering tool that can be used to characterize the pollutants to trace the contributions of pollutants from different sources within the mixing zones of estuaries, coastal water, and shelf water [30].Like other coastal regions of the world, the Karachi coast, especially the Manora Channel, is heavily polluted due to untreated industrial wastewater and Metropolitan municipal sewage, which are indiscriminately discharged into coastal waters through Layari and Malir rivers [68]. According to a report [32], only 20% of total annual wastewater produced in Metropolitan Karachi is treated and the rest is discharged directly into coastal waters. Sea pollution is further enhanced due to oil spills from cargo ships and oil tankers. The dredging of channel round the year also adds pollution to coastal water in terms of suspended sediments load. This situation demands to characterize the coastal water in order to determine pollution load, its extent, and type. Past attempts to investigate the pollution load of Karachi coastal water [31, 5, 52, 54, 57, 67] were only confined to the use of physicochemical and hydrological techniques. Very recently, [2], however, attempts have been made to apply nuclear techniques to study radionuclide pollution along Pakistan coast. The present study was aimed to apply stable isotope techniques in conjunction with classical pollution monitoring tools to characterize the pollution type and its transport, the mixing of pollutants with sea water, and the origin of salinity in coastal aquifer.
COASTAL AREAS OF PAKISTAN
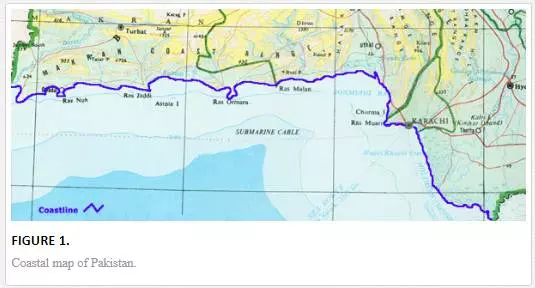
A 990-km-long strip of coastal area of Pakistan is stretched from Southeast (Ron of Katch) to Northwest (Gwader) along the Arabian sea [28]. It is geographically divided into two main zones, Baluchistan coastal belt (745 Km) and Sindh coastal belt (245 Km), as shown in Figure 1. Sindh coastal belt includes Indus Delta and Karachi Coast. The Baluchistan coastal belt is scarcely populated and is relatively pollution free. Sindh coastal belt, however, suffers very serious environmental problems because of greater population and industrial activities in Metropolitan Karachi, which is the largest city of Pakistan and is located at latitude 24° 48´ N and longitude 66° 59′ E on the coast of the Arabian Sea.
DESCRIPTION OF POLLUTION SOURCES OF KARACHI COAST
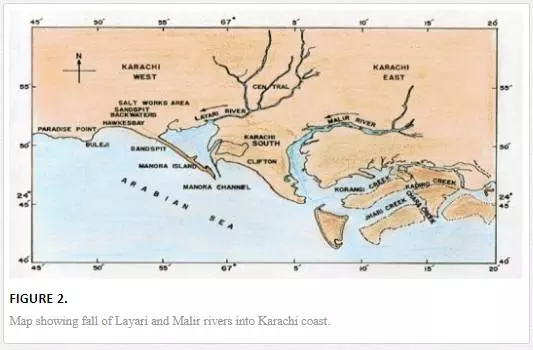
Metropolitan municipal sewage and industrial effluent are two major sources of coastal water pollution. The untreated effluent of more than six thousand industrial units scattered in six big industrial estates along with 300 Mgd municipal wastewater is discharged into Karachi coastal waters through Malir and Layari rivers [67]. Layari River passes right through the center of the Karachi Metropolitan while Malir river flows mainly through eastern part of the city (Figure 2). Both rivers act as an open sewage drain, receiving highly polluted wastewater of industrial and domestic origin. In accordance with a report [32], the Layari River discharges 130,000 tons of solid nitrogen, 160,000 tons of organic matter, 800 tons of nitrogen compounds, 90 tons of phosphate compounds, and 12,000 tons of suspended solid every year in the Manora Channel.
STABLE ISOTOPE TECHNIQUES
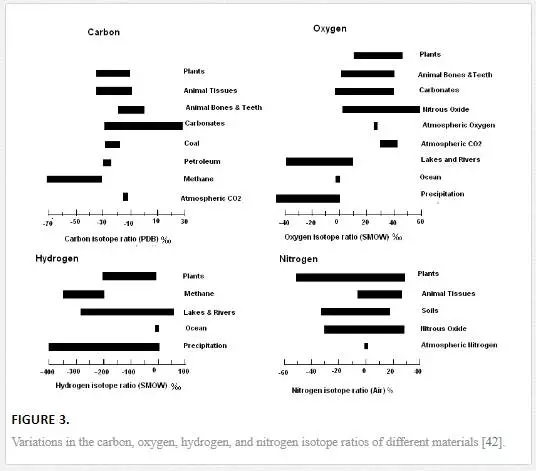
Naturally occurring isotopes are found in both stable and radioactive forms in the environment and are known as environmental isotopes [13]. Isotopes of an element exhibit similar chemical properties but differ in physical properties. The difference in the physical properties (bond strength and velocity) gives rise to fractionation among isotopes of an element in an environmental matrix. Fractionation takes place by natural processes, such as evaporation, condensation, and diffusion (kinetic isotope effects), or by ordinary mixing processes. Isotope fractionation leads to isotope variation, which makes it possible to use isotopes as tracers for the study of diverse nature of pollution problems [16, 22, 25, 39]. Figure 3illustrates the natural variations of 18O, 13C, 15N, and 2H in environmental matrices. Stable isotopes such as isotopes of light elements (hydrogen, carbon, nitrogen, oxygen, sulfur, and chlorine) in combination with conventional techniques are most commonly used in the environmental studies [30, 40, 43, 58].
Objectives of the study
The main objectives of the study were as follows:
1. To establish baseline inventories of stable isotopes (carbon, sulfur, hydrogen and oxygen), chemical parameters (physicochemical, heavy metal) in marine coastal waters, and/or sediments along Karachi coast for pollution monitoring
2. To determine the extent of mixing of polluted waters of Layari and Malir rivers into the Karachi coastal waters using stable isotopes of carbon (13C), oxygen (18O), and sulfur (34S)
3. To study the potential of stable carbon (δ13C) and nitrogen (δ15N) compositions of seaweeds and mangroves as pollution tracers in coastal area of Karachi
4. To determine potable water quality and to identify sources and dynamics of groundwater salinization of Karachi coastal aquifer using stable isotope of oxygen (δ18O) and hydrogen (δD) along with hydrochemical data
Materials and methods
LOCATION OF SAMPLING SITES
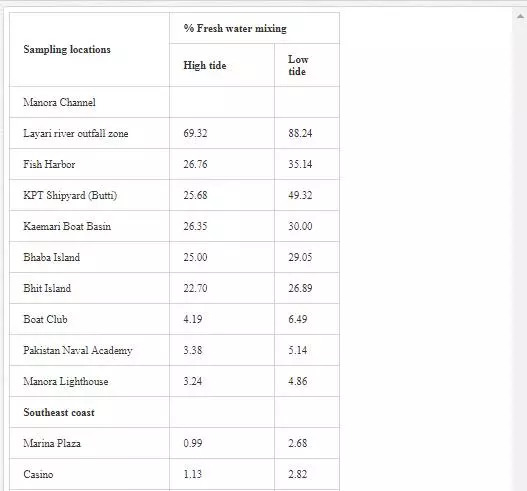
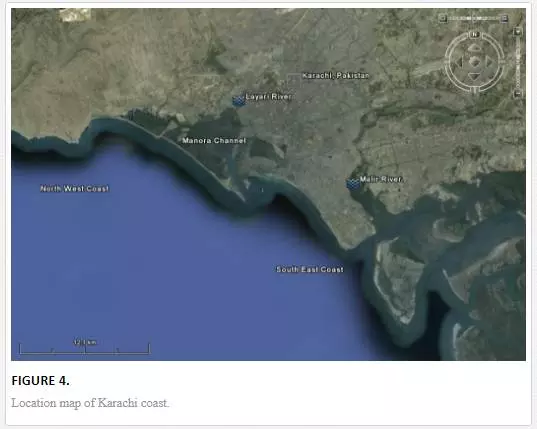
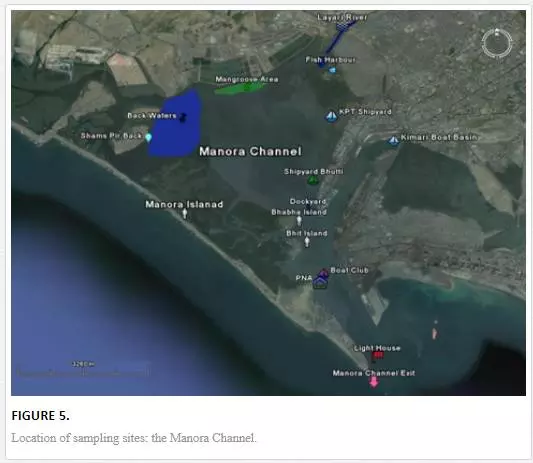
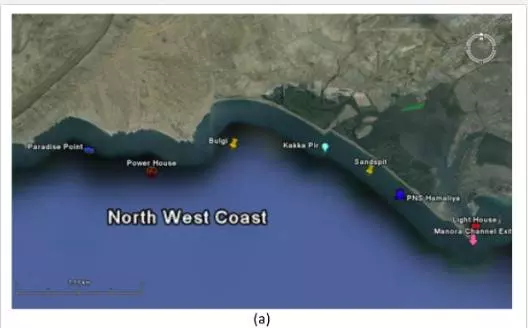
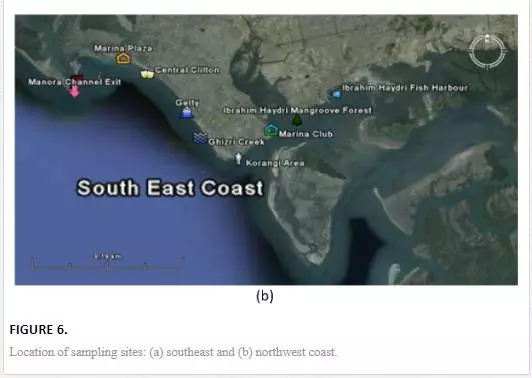
For sampling, Karachi coast was broadly divided into three zones: (i) Manora Channel, (ii) southeast coast, and (iii) northwest coast (Figure 4). Seawater from the Manora Channel was sampled from several sites, including Layari River outfall zone, Fish Harbor, KPT Shipyard Butti, KPT Shipyard, Kaemari Boat Basin, Bhaba Island, Bhit Island, Boat Club, Pakistan Naval Academy, and Manora Lighthouse (Figure 5). Sampling sites along the southeast coast include Marina Plaza, Casino, Naval Jetty, Marina Club, Ghizri area, and Ibrahim Haideri Fish Harbor, whereas along the northwest coast, sampling sites are Manora island sea side, PNS Himalaya, Kakka Pir, Buleji, Power house, and Sunari point. Figure 6 shows the locations of these sites. The location of sampling points was determined with Garmin GPS-100 Personal NavigatorTM (M/S Garmin, 11206 Thompson Avenue, Lenexa, KS 66219).
SAMPLE COLLECTION AND PRESERVATION
The period of field sampling was spanned over 2 years (April 2002–September 2004). This period was selected in order to cover overall variation in pollution transport pattern due to monsoon system. To investigate invasion of seawater, water samples were collected from Layari River, Malir River, Indus river, Hab dam, Karachi sea, shallow aquifer (depth <50 m), and deep aquifer (depth >50 m). All the water samples were preserved in accordance with standard procedure [51, 45, 64].
CHEMICAL ANALYSIS
Samples of seawater, river water, and groundwater were characterized in terms of electrical conductivity (EC), turbidity, and pH.Turbidity was measured with a portable turbidity meter. Each instrument was duly calibrated before use. Major ions were Cl-, SO4-2, and HCO3-1. Chloride contents were determined by ion selective electrodes with Orion Microprocessor Ion Analyzer/901. Carbonate and bicarbonate contents were determined by titration. Sulfate concentrations were determined by a spectrophotometric method using Hitachi 220-A Double Beam Spectrophotometer. Standard procedures [977.15 and 986.15 methods] after [34] and [7] were used for metal analysis of seawater and sediment by atomic absorption spectrophotometer and inductive couple plasma optical emission spectrometer. Metal analyses were performed using inductive couple plasma optical emission spectrometer (ICP-OES, Model 3580).
STABLE ISOTOPE ANALYSIS
The stable isotope analyses were performed using a modified Varian Mat GD-150 Mass Spectrometer. Stable isotope data are reported as standard mean ocean water (SMOW) for 18O and 2H analyses, Pee-Dee Belemnite (PDB) for 13C analysis of total dissolve inorganic carbon, and Canyon Diablo Troilite (CDT)for 34S. The overall analytical errors are ± 0.01‰ for δ13C, ± 0.1‰ for δ18O and δ34S, and ±1‰ forδ2H measurements. To ensure precision, the standard deviation of the mass spectrometer was also computed, and the standard deviation of each sample was ensured to be within permissible limit. For isotope analysis on mass spectrometer, water/sediments/plant samples are converted into gas phase. Since different sample preparation systems are used for analysis of C, S, N, O, and H, these systems were accordingly modified/redesigned in laboratory.
DETERMINATION OF POLLUTED WATER MIXING WITH COASTAL WATER
A two-component isotope mixing equation was used to compute polluted river water mixing with coastal water. The mixing fraction (f) of polluted Layari river water (source A) and/or Malir river water (source A) with nonpolluted seawater (source B) of Karachi coast is computed as follows:
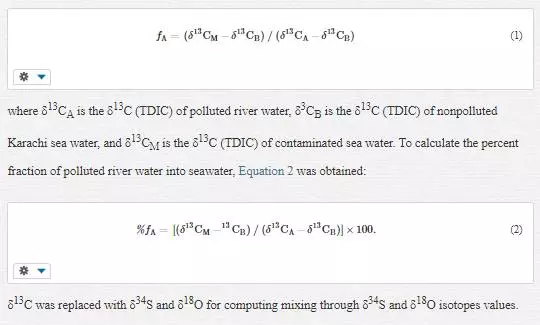
Results and discussion
CHEMICAL CHARACTERISTICS OF COASTAL WATER AND SEDIMENTS
Heavy metals are considered as one of the hazardous pollutants in natural environment due to their toxicity, persistence, risk (direct/indirect) to human beings and aquatic life [1, 10, 11, 62], and long-term damage to the environment [21, 62]. Metal elements are added to water bodies of our environment either through natural processes and/or anthropogenic activities. Heavy metals like Cu, Cr, Ni, Mn, and Zn are listed among metals known to be essential for aquatic life [59]. However, these metal elements have well-known toxic effects if they are present above permissible limits [62]. Heavy metal load in seawater/sediments is especially measured in this study because of their continuous addition to seawater due to the indiscriminate discharge of municipal sewage and industrial effluent through Layari and Malir rivers [8, 37, 38, 46, 68].
HEAVY METAL CONCENTRATION OF POLLUTED RIVERS AND COASTAL WATERS
Heavy metal levels in Layari and Malir river water and Karachi coastal waters are summarized in Table 1. Cr contents of Malir river water are relatively higher as compared to Layari River, which could be due to a continuous discharge of untreated effluents of tannery industrial units into Malir River. Higher Cu and Zn concentrations in Layari River can be explained best due to the influx of wastewater of industrial units of cable, electrical appliances, electroplating, textile, and glass [36]. Elevated levels of Ni and Mn in water of Layari and Malir rivers owe to the inflow of untreated effluents such as automobile batteries, electroplating, car painting dying, and glass industries [67].
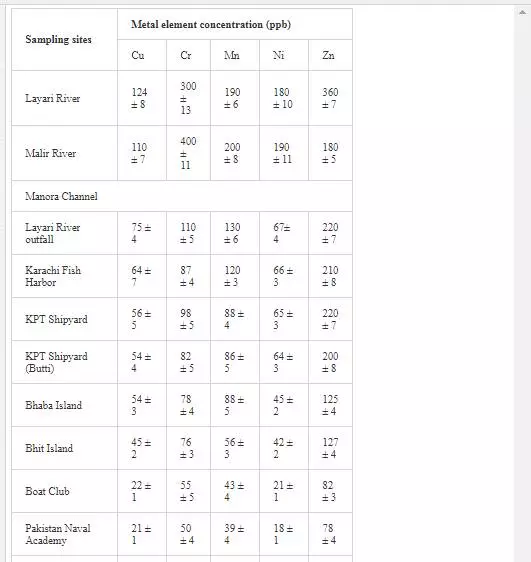
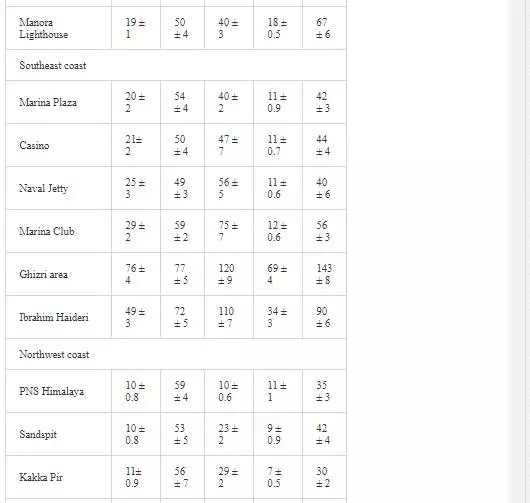
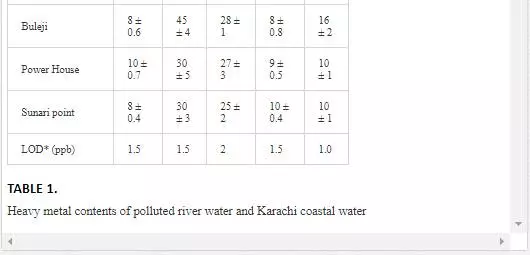
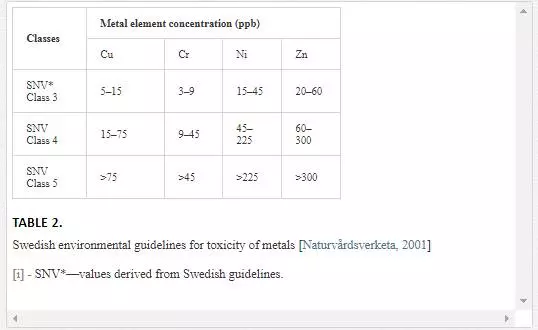
Swedish guidelines for safe seawater for aquatic life are divided into three broad classes depending upon the degree of environmental disturbance (Table 2). Class 3 shows safe limit of heavy metal content in seawater. Class 4 indicates an increased risk of environmental disturbance, and class 5 represents a high risk of environmental disturbance even at shorter exposure.
HEAVY METAL CONTENTSOF SEA SEDIMENTS
Surficial sediments are a feeding source for biological life, a transporting agent for pollutants, and an ultimate sink for organic and inorganic matter settling. In heavily polluted sediments, the anthropogenically introduced components by far exceed the natural components and pose a risk to the marine ecosystem [4, 66]. Many researchers [5, 31, 36, 55, 56] attempted to evaluate heavy metal pollution of Karachi coastal sediments. Heavy metals in sediments are generally much higher as compared to seawater because heavy metals entering from other sources into seawater ultimately deposit onto sediments [19, 41]. As shown in Table 3, Mn and Ni contents in coastal sediments of Karachi are much higher than EPA guidelines at all sites except at one site (Kakka Pir).
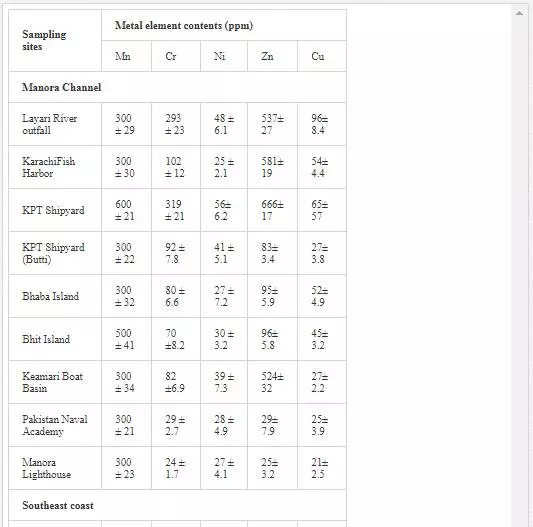
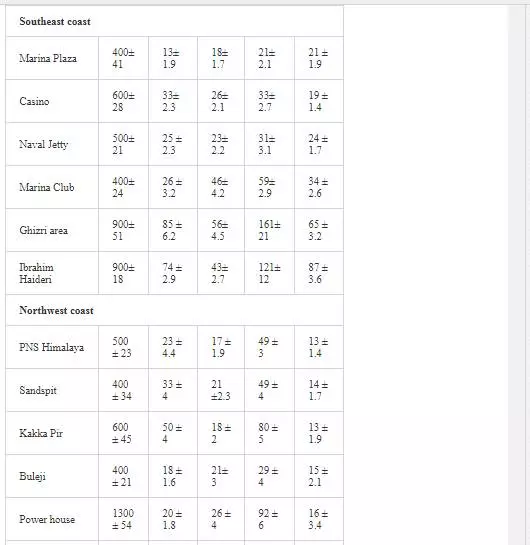
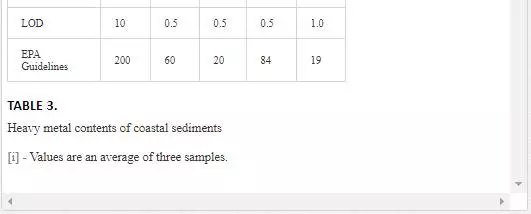
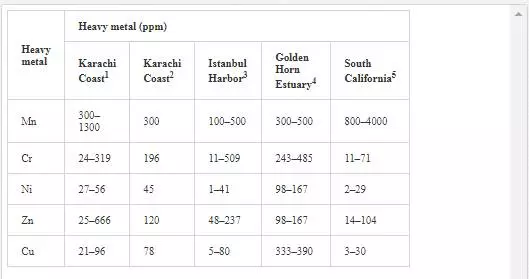
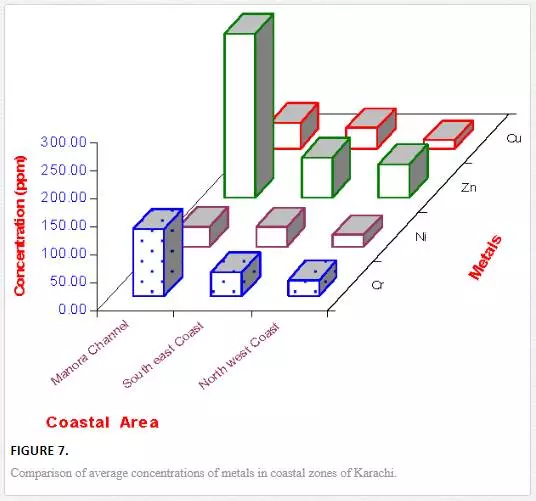
In sediments of the Manora Channel, levels of Cr, Zn, and Cu are also higher except at Manora Lighthouse and Pakistan Naval Academy. Heavy metal load also appeared to be decreased as the distance of sampling site is increased from the entry point of Layari River into the Manora Channel. However, exceptionally higher levels are recorded at KPT Shipyard sediments, which may be due to submarine disposal of effluent of KPT Shipyard in addition to untreated domestic and industrial effluents entering the sea. Layari River outfall zone and Karachi Fish Harbor sediments also show considerably high levels of heavy metals, which owes to wastewater of industrial activity such as boat building and fish processing at the harbor. The Manora Channel sediment is clayey, which has high absorption or trapping capacity for heavy metals [62]. Heavy metal load in southeast coastal sediments is considerably less as compared to sediments of the Manora Channel. Metal contents are appeared to be substantially higher than EPA guidelines at the Ghizri area and Ibrahim Haideri due to continuous influx of untreated domestic and industrial waste into the sea at these sites. A comparison of heavy metal concentrations of three coastal zones is given in Figure 7, which shows that sediments of the Manora Channel are heavily contaminated as compared to southeast and northwest coastal sediments. This may be due to the heavy influx of untreated municipal wastewater and industrial effluent into the Manora Channel. A comparison of heavy metal contents of Karachi coastal sediments with other coastal sediments of the world (Table 4) reveals that the findings of this study are in agreement with the results of a previous study [36] and other studies of the world with an exception of South California harbor sediments where Mn concentration is many times higher than other sea sediments and other metal element contents are significantly low as reported in the other studies
STABLE ISOTOPE CHARACTERISTICS OF KARACHI COASTAL WATER, SEDIMENTS, AND BIOTA
Stable isotope analysis of coastal water, sediments, and biota was undertaken in order to understand the mechanism of the land base terrestrial pollution transport to coastal ecosystem.
Δ13C (TDIC)ANALYSIS OF POLLUTED RIVERS AND COASTAL WATER
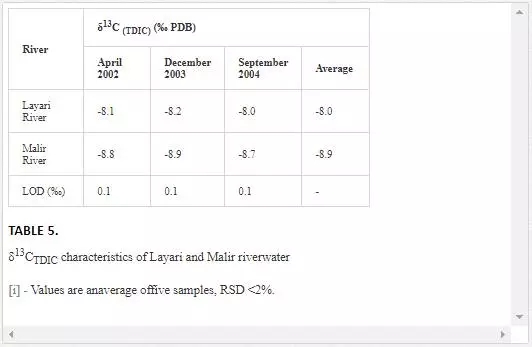
Theδ13C(TDIC) value for normal seawater is generally in the range of ±1 per mill PDB [13], andδ13C(TDIC) values of waste drains are in the range of-16 to -10 per mill PDB [23, 24].As shown in Table 5, Layari and Malir river water is depleted in δ13C(TDIC).The depleted values of δ13C(TDIC)in water of Layari River(- 8.2‰ PDB) and Malir River (-8.8‰ PDB) owe to the heavy influx of sewage.
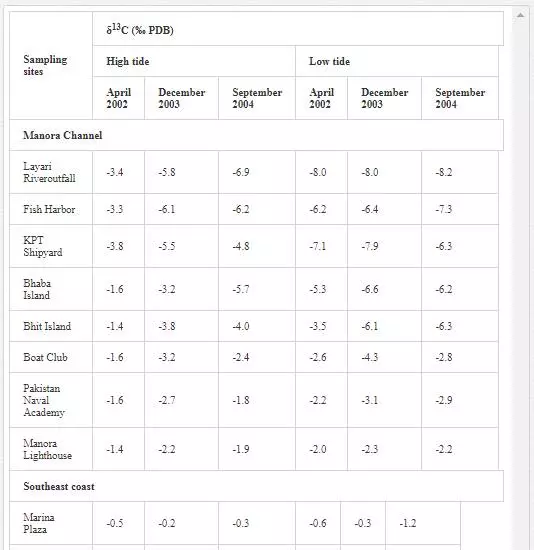
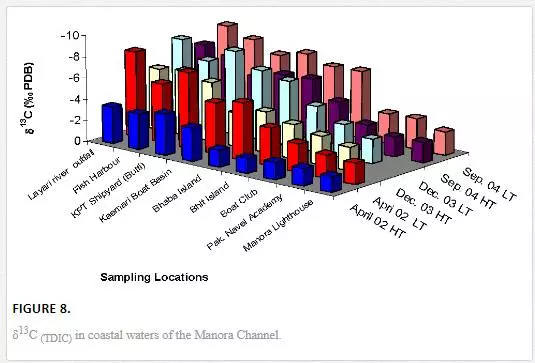
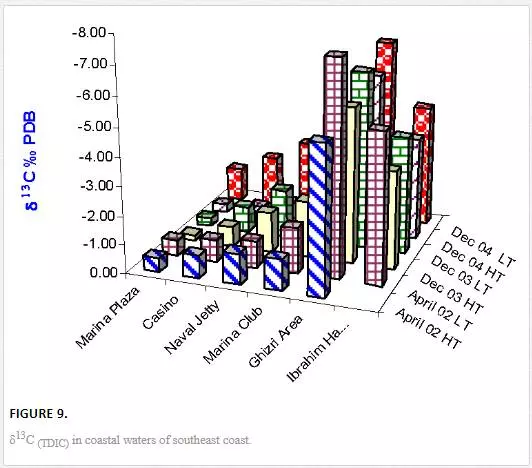
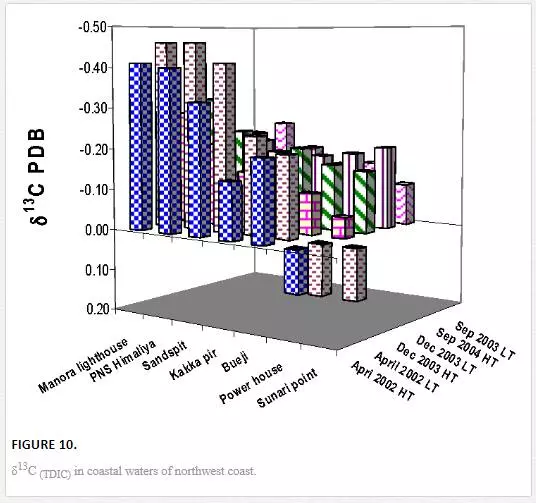
δ3CTDIC value is a function of sewage pollution load and can be used in tracing land based pollution flow in marine water. Numerous researchers [12, 23, 26, 27, 61] have used δ3C as a tool to track sewage pollution in the aquatic environment. This study shows that variations in δ13CTDIC contents of the Manora Channel seawater are strongly associated with tide conditions, distance from the entrance of Layari River into the sea, and seasonal variation (Table 6). It is apparent that the Manora Channel water is relatively less depleted in δ13CTDIC during high tide as compared to low tide conditions, which means that high tide environment retards the mixing of the municipal sewage into seawater. Hence, channel water is relatively more depleted in δ13CTDIC during low tide as it facilitates the diffusion of sewage pollution with seawater. The diffusion of sewage pollution appears to be gradually decreased as the distance of sampling site is increased from Layari River joining point with seawater and is shown by a progressive reduction in δ13CTDIC values (Figure 8).The δ13CTDIC values of seawater at PNA and Manora Channel Lighthouse are close to δ13CTDIC values of normal unpolluted seawater, indicating less influx of sewage. Seasonal variation in δ13CTDIC values is attributed to strong currents and high flow of seawater in summer, which hinders the mixing of sewage pollution as indicated by relatively less depleted values of δ13CTDIC as compared to weak currents and moderate seawater flow in winter. Southeast coastal water is enriched in δ3C (TDIC)as compared to the Manora Channel, which indicates less influx of sewage pollution. However, considerable depleted values of δ3C (TDIC) are observed at the Ghizri area where Malir River empties its pollution load (Figure 9). However, seawater of northwest coast exhibits fairly constant δ13CTDIC values (-0.2‰ to 0.46‰ PDB), which are close to normal seawater value and appears to be independent of tidal and seasonal effect (Figure 10). It indicates substantially low sewage pollution load as compared to the Manora Channel and the southeast coast.
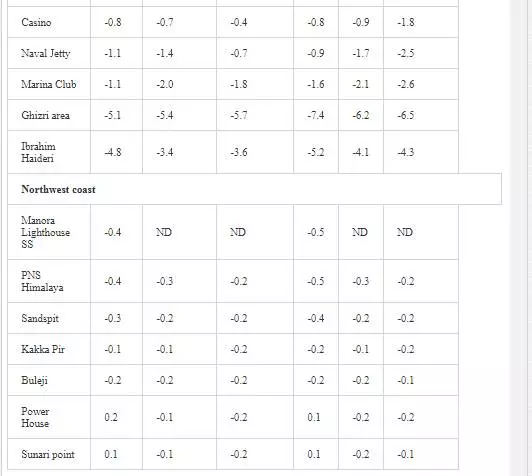
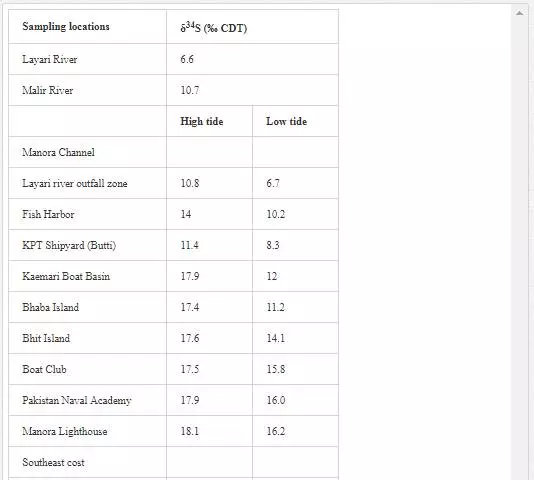
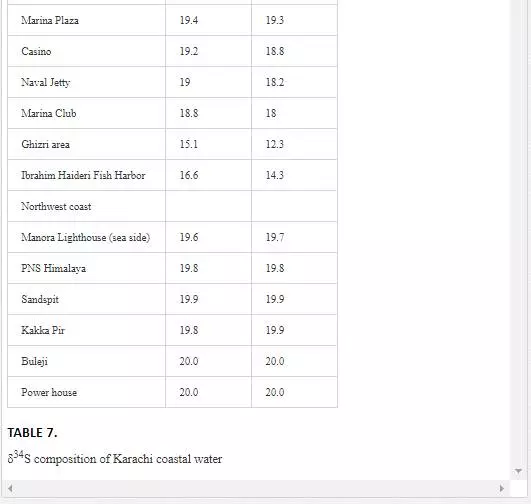
STABLE SULFUR (Δ34S) ANALYSIS OF POLLUTED RIVERS AND COASTAL WATER
Many researchers [3, 35, 48, 49, 50, 63] have applied δ34S content of seawater for evaluating sewage pollution contribution toward marine ecosystem. The δ34S value of normal seawater is around 20‰ CDT, and the seawater is depleted in δ34S with the addition of sewage wastewater. Sampling for δ34S was performed only in April 2002. Layari and Malir river waters (6.6‰ and 10.7‰ CDT respectively) are notably depleted in δ34S (Table 7) because of municipal wastewater (sewage pollution). The δ34S composition of coastal water of Karachi Metropolitan is used to ascertain the seawater contamination sources. It is also evident from Table 7 that the factors controlling variations in δ34S composition of the Manora Channel water are tide conditions and distance from the confluence point of Layari River with sea. The Manora Channel water is enriched in δ34S during high tide as compared to low tide conditions because high tide conditions hamper the mixing of sewage with seawater. During low tide, however, channel water is relatively less enriched in δ34S as it facilitates the mixing of sewage pollution with seawater. Sewage pollution gradually decreases as the distance of sampling site is increased from Layari River entry point with seawater. Southeast coastal waters are depleted in δ34S value at the entrance point of Malir River into seawater at the Ghizri area and Ibrahim Haideri Fish Harbor area. At other sampling sites, δ34S values of seawater are close to the normal seawater value, which demonstrates that the sewage pollution of Malir River diffuses quickly in coastal water due to strong open sea currents. δ34S values of northwest coastal water are apparent to be near to its value for normal seawater in both low and high tide conditions, which shows little domestic wastewater discharge along northwest coastal water.
STABLE OXYGEN (Δ18O) ANALYSIS OF POLLUTED RIVERS AND COASTAL WATER
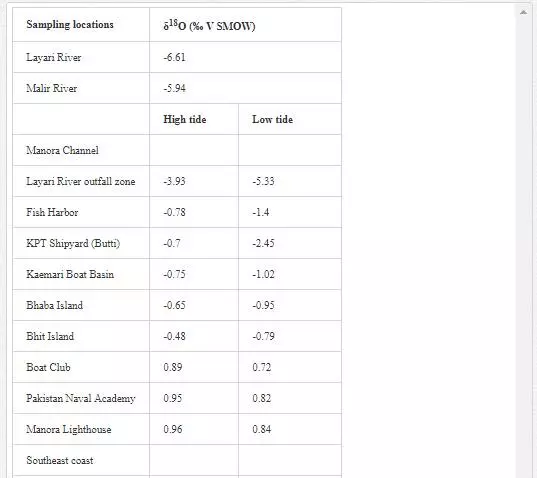
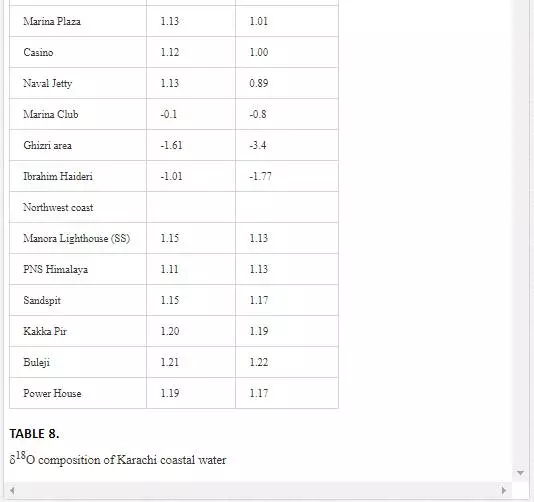
Oxygen isotope (δ18O) has been extensively used by several researchers [6, 29, Hudson et al. 1995;] to determine mixing of fresh water with seawater as their δ18O values are quite different. δ18O value for seawater is around 0‰ [Lear et al., 2000], while freshwater shows a wide range of δ18O isotopic compositions, generally lower than -5‰. However, seawater is depleted in δ18O with the mixing of fresh water [13]. Sampling for δ18O was performed in April 2002. As shown in Table 8, Layari and Malir rivers have δ18Ovalues of -6.61‰ and -5.94‰, respectively, and are in the range of freshwater values. The δ18O composition of seawater also varies with sea tides and how far a sampling site is from the points where these rivers empty wastewater into sea. In the Manora Channel, seawater appears to be depleted in δ18O where fresh water, discharged through Layari River, is mixed with the seawater (Layari River outfall zone). However, seawater is found gradually enriched in δ18O with increasing the distance from the joining point of Layari River with sea, with an exception of KPT Shipyard where δ18O values is -2.45‰ during low tide environment. Low values of δ18O could be due the mixing of KPT Shipyard wastewater. Southeast coastal seawater is less depleted in δ18O as the Malir river wastewater disperses into seawater quickly. However, at Ghizri area, which is entry point of Malir River into the coast, seawater is depleted in δ18O. Along northwest coast, δ18O values of seawater remains constant around 1‰ and appears to be independent of tide conditions.
STABLE ISOTOPE ANALYSIS OF MARINE BIOTA
Isotope signatures δ13C and δ15N of mangrove and seaweeds from different coastal locations were determined to see possible effect of land based pollution on marine plants in terms of their stable isotope composition and physiological characteristics.
Δ13C AND Δ15N ANALYSIS OFMANGROVES
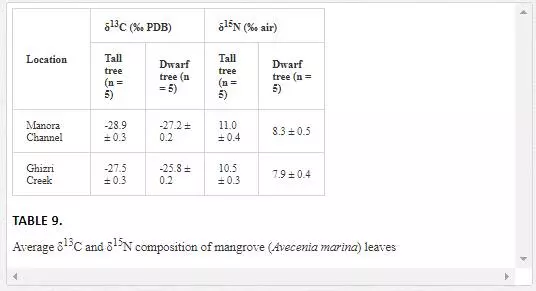
Stable carbon and nitrogen isotopes composition of mangroves are widely used in environmental studies [Lin and Sternberg, 1992; McKee et al. 2002; Kao et al., 2001]. For example, the leaves of tall and dwarf mangroves often have distinctly different carbon and nitrogen stable isotopic characteristics, which are related to the various environmental conditions such as nutrient status and water use efficiency [Alongi et al., 1992; McKee and Faulkner, 2000; Blasco and Saenger, 1996; Medina and Francisco, 1997]. Dwarf trees are associated with sites that are often in the interior of mangrove islands, while tall trees are, generally, found at the edge of islands. The morphological differences are indicative of phosphorus and nitrogen limitation [McKee et al., 2002].
δ13C values of mangrove leaves ranged from -28.9‰ to -25.8‰ PDB, which are typical of most C-3 plants [O’Leary, 1981]. The mangroves leaves of the Manora Channel backwaters (receiving domestic waste from Layari River) are depleted in δ13C as compared to δ13C of Ghizri Creek mangroves leaves growing in open sea environment (Table 9). McKee et al. (2002) also reported that mangroves leaves growing at domestic waste sites are depleted in δ13C. However, δ15N values of mangroves in the Manora Channel were quite comparable with δ15N values of mangroves found in the Ghizri area, which suggests that the δ15N levels of Mangroves is not a reliable indicator to differentiate between mangroves growing in polluted and nonpolluted sea sites.
Acomparison of δ13C values of tall and dwarf mangrove plants revealed that δ13C values of interior tree leaves are higher as compared to leaves of tall trees leaves. The plants with high δ13C values are reported to have higher water use efficiency [Lin and Sternberg, 1992]. Our results, thus, show that a dwarf mangrove population has higher water use efficiency as compared to tall trees. The findings are also in agreement with earlier study by Lin and Sternberg (1992), where it was shown that dwarf mangrove populations had higher water use efficiency than tall trees. It is generally assumed that water use efficiency is related to the stomatal conductance. Dwarf trees have a lower ratio of the intercellular to atmospheric CO2 resulting in an enriched δ13C value and a higher water use efficiency [Farquhar, et al., 1988; Wooller et al., 2003; Lin and Sternberg, 1992].
Δ13C AND Δ15NANALYSIS OF SEAWEED
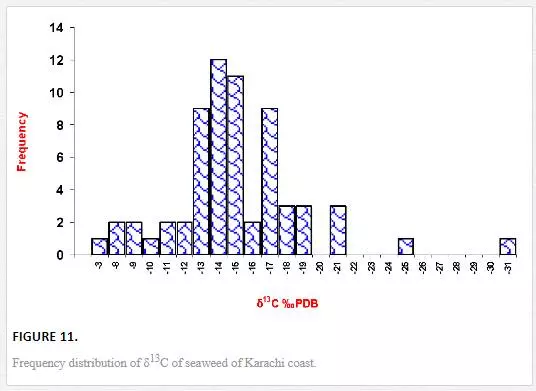
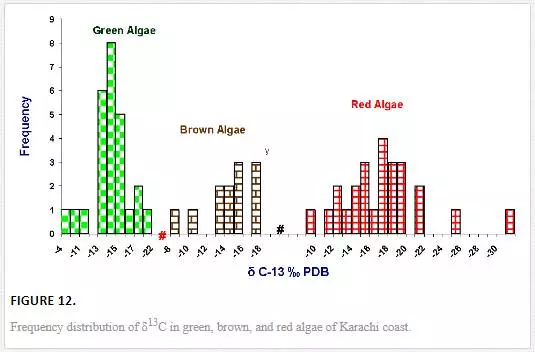
Stable carbon and nitrogen isotope compositions were determined in different types of seaweeds as well. δ13C values of seaweed ranged from -31.1‰ to -4.9‰ PDB. However, δ13C contents in majority of seaweeds were in the range of -19‰ to -13‰ PDB (Figure 11), which were in good agreement with the reported values for tropical and subtropical algae (Craig, 1953; Black and Bender, 1976; Fry, et al., 1982). δ13C frequency distribution histograms of green, red, and brown algae are represented in Figure 12. δ13C values of green algae were in the ranges of -21.14‰ to -4.9‰ PDB with a mean value of -14.1‰ PDB. δ13C values of brown algae were found to be in the range of -17.82‰ to -8.9‰ PDB with a mean value of -14. 4‰ PDB, whereas δ13C of red algae ranged from -31.12‰ to -9.49‰ PDB with a mean value of -17.38‰ PDB. The average δ13C content of red algae (-17.4‰) was low as compared to brown (-14. 4‰) and green algae (-14.1‰). Fry et al. (1982) and Maberly et al. (1992) also reported marine red algae with relatively high δ13C value. However, the highest δ13C value was recorded in the green algae Ulva fasciata (-4.9‰). Carbon isotope composition of terrestrial plants can be used to depict their photosynthetic pathways. The δ13C values for terrestrial C3, C4, and CAM plants range from -22‰ to -38‰, from -8‰ to -15‰, and from -13‰ to -30‰, respectively [Yeh and Wang, 2001]. Carbon isotope values of marine algae of Karachi coast ranged between -31.1‰ and -4.9‰. However, δ13C values for majority of seaweed were in the range of -19‰ to -13‰, which apparently corresponds to terrestrial C4 plants. It is reported in the literature that only one seaweed (Udotea flabellum) is known to have C4 like photosynthesis, while majority of seaweeds are C3 plants [Reiskind, et al., 1999: Wang and Yeh, 2003]. Thus, seaweed cannot be clearly categorized into C3 or C4 plants on basis of δ13C composition. This can best be explained by the fact that δ13C values of seaweed may be changed due to extent of CO2 diffusion or HCO3- active transport process. For marine algae, two carbon sources are available for photosynthesis: CO2 and HCO3-. However, it is difficult to guess what proportion of the different carbon sources (HCO3-1 and CO2) are taken up by the different seaweeds, which obviously complicates the interpretation of the data.
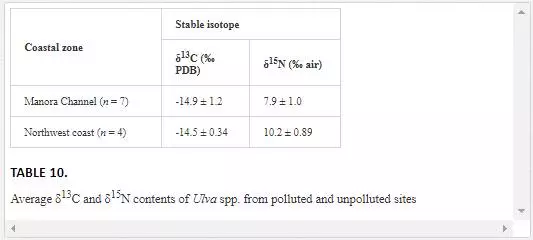
To assess the effect of domestic waste on isotope composition of seaweed, δ13C values of Ulva spp. from contaminated (Manora Channel) and uncontaminated sites (northwest coast) were compared. Average δ13C values in Ulva spp. from polluted site (-14.9‰) and nonpolluted site (-14.5‰) do not display any significant isotopic shift, suggesting that δ13C composition of Ulva spp. was not influenced by domestic wastewater (Table 10). This may be the result of increased primary productivity and/or the lower availability of dissolved inorganic carbon. Ulva spp. is green algae that fix atmospheric carbon by photosynthesis and does not utilize dissolved inorganic carbon from dissolve inorganic pool (DIC). Hence, it can be concluded that δ13C cannot discriminate Ulva spp. of polluted and nonpolluted site. These results agree well with the finding of Rogers (1999), where he observed that Ulva spp. growing in polluted site was slightly depleted in δ13C as compared to uncontaminated site but difference was not significant.
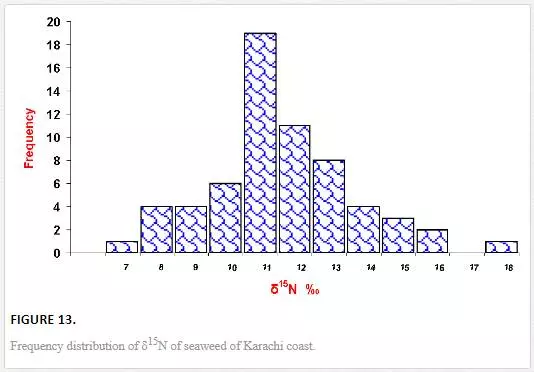
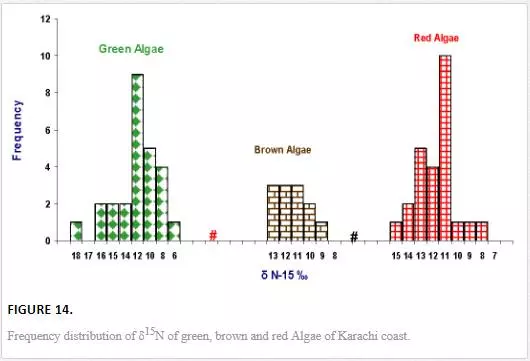
Unlike δ13C, variation in δ15N values of seaweeds was small and ranged from 6.1‰ to 17.8‰ air (Figure 13).δ15N values of green algae varied between 6.1‰ and 17.7‰ air with an average value of 11.0‰ air, brown algae ranged from 8.9‰ to 12.9‰ air with an average 10.8‰ PDB, and δ15N concentration of red algae was in the range of 7.7‰ to 14.5‰ air with an average value of 11.2‰ air (Figure 14). Red algae were enriched in δ15N as compared to brown and green algae. δ15N values of Ulva spp. from both polluted, and unpolluted sites are compared in Table 11. The average δ15N values (10.2‰ air) of Ulva spp. from relatively clean water of northwest coast was enriched in δ15N as compared to average δ15N content (8.0‰ air) of same species collected from the Manora Channel (polluted water). The δ15N values of the Ulva spp. (10.2‰ air) from the northwest coast (nonpolluted site) are close to δ15N values of nonpolluted seawater nitrate. This indicates that Ulva spp.utilizes nitrate of nonpolluted seawater, similar results are quoted by Monteiro et al. (1997) and Peterson et al. (1985). δ15N values (8.0‰ air) of Ulva from polluted the Manora Channel are close to δ15N values of sewage, indicating that Ulva of polluted site absorb and assimilate sewage-derived nitrogen. Several researchers [Cabana and Rasmussen, 1996; Monteiro et al., 1997; Hobbie and Fry, 1990; Udy and Dennison, 1997] have also reported that seaweed exposed to sewage discharge are depleted in δ15N. Thus, it can be inferred that nitrogen isotope of Ulva spp. could be a good indicator of sewage pollution.
STABLE CARBON ISOTOPE COMPOSITION OF SEA SEDIMENTS
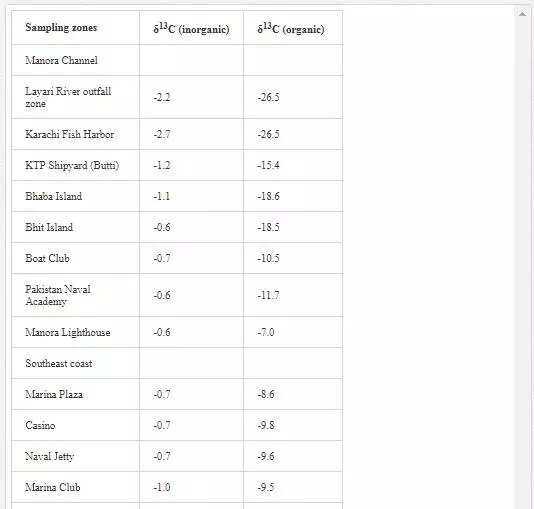
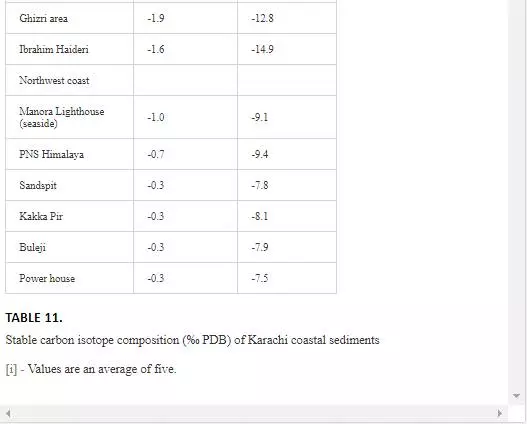
δ13C contents (inorganic and organic) of sediments from Karachi coast are shown in Table 11. δ13Cinorgvalues of Manora Channel sediments varied from -2.7‰ to -0.6‰ PDB, and δ13Corg contents were in the range of -26.5‰ to -7.0‰ PDB. The sediments from Layari River and Karachi Harbor were appeared to be extremely depleted in δ13Corg (-26.5‰), which indicated domestic sewage as the main source of these sediments. Another reason could be the lithology of the sediments, which was predominantly clay and organic carbon, had affinity toward argillaceous materials [Augley, et al., 2007]. Descolas-Gros and Fontugne (1990) also reported similar δ13Cinorg values in the sediments originating from domestic wastewater. δ13Corg contents of sediments of southeast coast were in the range of -14.9‰ to -8.6‰ PDB. These values do not show any link with sewage material and indicated that sewage material carried through Malir River is diluted and dispersed by sea waves. However, low δ13Corg values recorded in the sediments of Ghizri (-12.86‰ PDB) and Ibrahim Haideri (-14.9‰ PDB) might represent organic matter of dead phytoplankton [Descolas-Gros and Fontugne, 1990]. δ13Cinorgvalues of sediments pertaining to northwest coast were in the range of -1.0‰ to -0.3‰ PDB, and δ13Corg ranged from -9.4‰ to -7.5‰ PDB, indicating no domestic waste input in sediments.
TRITIUM
Tritium (3H) is isotope of hydrogen with relatively short half-life (12.3y) and decays with an emission of a beta particle[Martin, 2000]. Tritium was introduced into the atmosphere primarily as a result of nuclear bomb testing from the period 1951 to 1957. The natural concentration of tritium in seawater is around 0.02 Bq kg-1. However, its concentration up to 100 Bq kg-1 has also been reported in some seawaters [McCubbin and Leonard, 2001].
The most important use of tritium is in distinguishing water that entered in water bodies prior to 1952 from water that entered into water bodies after 1952 [Drever, 1997]. Tritium is also used for studying mixing of seawater with fresh water [Mazor, 1991]. As shown in Table 12, tritium (3H) content was higher in water of Layari (7.3 TU) and Malir (7.2 TU) river water as compared to seawater where tritium was in the range of 0.8 – 6.0 TU. Tritium content of seawater in mixing zone of Layari River outfall area was 6.0 TU, which decreased as the increased distance from the mixing zone toward Manora Channel exit (1.6 TU at Manora Lighthouse). Tritium contents in Manora Channel water seawater were higher than southeast and northwest coastal water, which show mixing of Layari River in seawater of Manora Channel.It has also been reported that tritium content >10 TU in a water sample indicates a post-1952 water, whereas a tritium content lower than 0.5 indicate a pre-1952 water. Tritium content in between 0.5 and 10 represents a mixture of pre-1952 and post-1952 water [Mazor, 1999; Clark and Fritz, 19971]. Tritium contents of Karachi coastal water are between 0.8 and 6.2, which suggested that Karachi seawater is a mixture of pre-1952 and post-1952 water.

Computation of polluted river water mixing with seawater using stable isotopes
A two-component mixing equation was used to assess the pollution contribution to seawater by the Metropolitan wastewater by using stable carbon and sulfur isotopes in addition to the application of oxygen isotope ratio to determine fresh water mixing with seawater. When δ13C and δ14S depleted Malir and Layari river water enters into δ13C and δ34S enriched seawater, the resulting isotopic signature of mixed water is different from both mixing water bodies. Thus, isotopic values of the two end points (such as polluted water body and seawater) can be used to estimate the contribution of polluted river to marine water through a two-component isotope mixing equation [Spiker, 1980]. Numerous researchers [18; Balesdent, et al., 1988; Amundson and Baisden, 2000; Vitousck, et al., 2003; Currie, 2007;[44]; Iqbal, 1998; Wels, et al., 1990; Raymond and Bauer, 2001; Fry, 2002] round the globe have documented the use of stable isotopes in a two-component mixing equation for various applications, including sewage flow in aquatic environment, food web studies, contribution of C-3 and C-4 plant sources to soil organic carbon, and contribution of different water sources in lake/stream. In this study, an attempt has been made to estimate mixing percentage of polluted water in marine environment through stable isotope signatures.
COMPUTATION OF RIVER WATER MIXING INTO KARACHI COASTAL WATER USING Δ13C (TDIC)
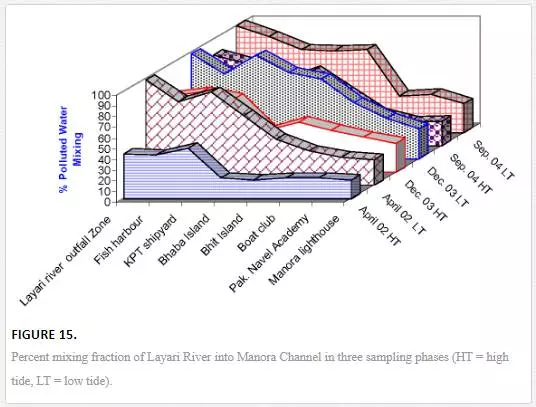
The equation described in Section 3.9 was used to quantify percentage fraction of Layari river water in Manora Channel water and Malir river water in southeast coastal water during the low and high tide conditions of the sea. The dominant factors that appear to control the extent of terrestrial pollution mixing with Manora Channel seawater are tide conditions, distance from the joining point of Layari River with sea and sampling season. High tide environment hinders the Layari river water mixing with seawater coupled with a gradual decrease in pollution levels from entrance point of Layari River into sea. The mixing of river water with seawater is substantially high near Layari River outfall zone and gradually lowers toward Manora Channel exit with the exception at KPT and Bhaba Island where the addition of sewage input from local population may be considered responsible for increased pollution load. However, in high tide environment, considerable mixing (%) of river water in Manora Channel seawater indicated incomplete flushing, which owes to the fact that Manora Channel is a semienclosed bay. Mixing pattern is also appeared to be effected by seasonal environmental variations. In April, strong currents and high flow of seawater in Manora Channel diluted the Layari River added pollution as compared to weak current season and moderate seawater flow in the channel during September and December when volume of seawater in the channel is decreased; thereby, the ratio of Layari river water to seawater in channel is increased. However, at Layari River outfall zone, mixing seems to be independent of seasonal variation, which is clear from the fact that fraction of Layari River in seawater remains more than 97% in all sampling seasons (Figure 15).
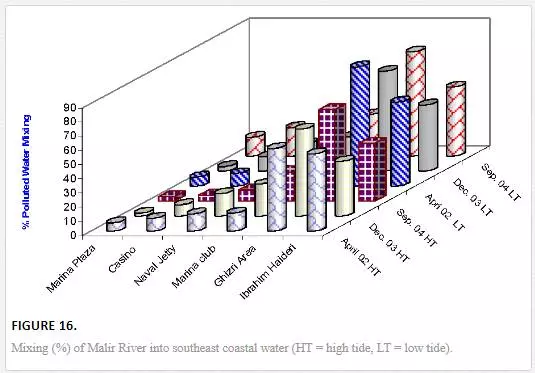
Mixing of Malir River with seawater is considerably high at the Ghizri area, which is the entrance point of Malir River in the sea. The pollution load diffuses quickly as the distance is increased from entrance point to other sampling sites. Under low tidal conditions, the Ghizri area and Ibrahim Haideri Fish Harbor contained 70–74% and 46–59% water of Malir River. However, at sampling sites like Plaza, Casino, Naval Jetty, and Marina Club, mixing Malir river water into seawater reduced to 5–29%, which shows quick dispersion of Malir water in the sea by strong open sea currents (Figure 16). It appeared that mixing pattern is not much effected by seasonal changes during low and high tide environments except at Ghizri area.
COMPUTATION OF POLLUTED RIVER WATER MIXING INTO KARACHI COASTAL WATER USING Δ34S VALUES
To estimate the percent fraction of polluted river water into coastal water using δ34S values, the equation in Section 3.9 was modified by replacing δ13C with δ34S to get the following two-component isotope balance equation:
![]()
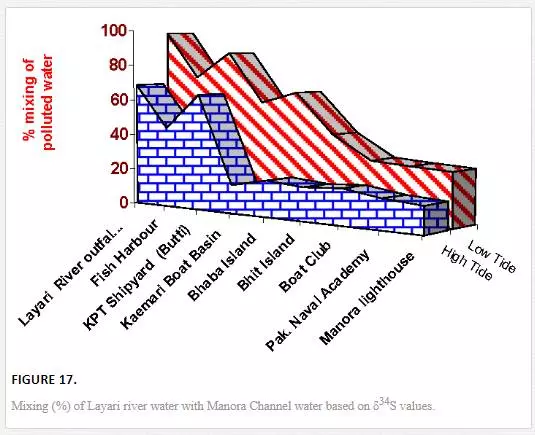
where δ34SA and δ34SB denotes stable sulfur isotope compositions of polluted river water and the nonpolluted seawaters, respectively. The mean δ34S values of 6.6‰ CDT and 10.7‰ CDT was taken for Layari and Malir river water, respectively, while assuming northwest coastal water as pollution free (20‰ CDT). Tide height and distance of sampling sites from the Layari River outfall are the main factors that play part in transport and spread of Layari River pollution in Manora Channel. Mixing of Layari water with sea water is low in high tide (14–69%) as compared to low tide (28–99%) conditions. There was an optimal mixing of river water with sea at Layari River outfall zone in high and low tide conditions. However, the fraction (percent) of polluted water in seawater appears to be gradually decreased from Layari River outfall zone to Manora Lighthouse (Figure 17).
The computation of Malir river water mixing with seawater computed through δ34S values show the increase in fraction (%) of river water with seawater was observed in low tide as compared to high tide environment particularly at the Ghizri area and Ibrahim Haideri Fish Harbor. However, at sites like Marina Plaza, Casino, Naval Jetty, and Marina Club, the increase in mixing (percent) was not very significant (Figure 17), which is attributed to dilution of Malir River pollution by open sea currents.
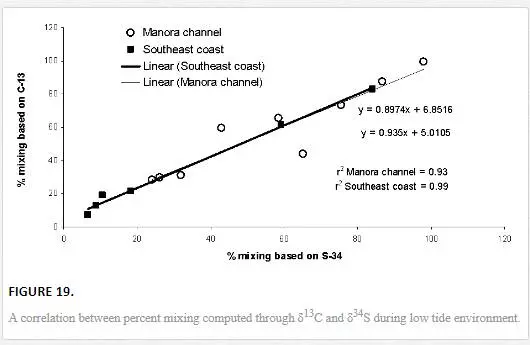
A COMPARISON OF MIXING COMPUTED THROUGH Δ13C AND Δ34S VALUES
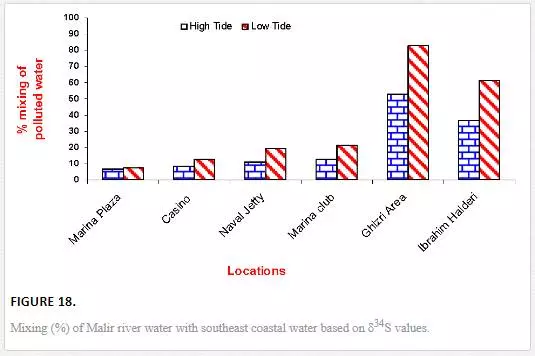
The results obtained through δ13C calculation were plotted against the δ34S calculation (Figure 18). Significant correlation coefficient in Manora Channel (r2 = 0.93) and southeast coast (r2 = 0.99) during low tide indicated that mixing calculation through δ34S and δ13C values supports each other and a strong relationship exists between the two isotopes for determining polluted water mixing with seawater.
APPLICATION OF Δ18O TO DETERMINE FRESHWATER–MARINE WATER MIXING
δ18O is a useful tool to define mixing between fresh water and marine water mixing [6, 29]. Some researchers [14, 18, 44] used δ18O contents in a two-component isotope mass balance equation for the calculation of mixing fraction of two sources in a water body.
In the present investigation, δ18O values of Layari and Malir river water, unpolluted sea water, and polluted seawater were used in the following two-component isotope equation to evaluate fresh water–seawater mixing:
%fA= [(δ18OMIX−δ18OB) / (δ18OA−δ18OB)]×100,%fA= [(δ18OMIX−δ18OB) / (δ18OA−δ18OB)]×100,
where fA is the fraction of river water in seawater, and δ18OA,δ18OB, and δ18O MIX stand for δ18O contents of river water, nonpolluted seawater, and polluted seawater, respectively.
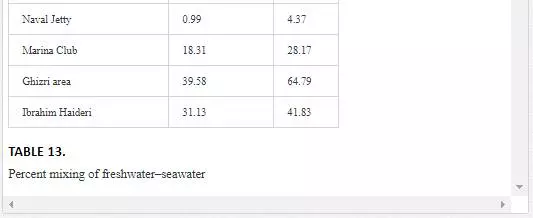
It is evident from Table 13 that freshwater–seawater mixing is influenced by tidal height and distance from the fresh water source. Low tide environment is favored, and high tide environment slows the mixing process. A progressive reduction in proportion of freshwater into seawater is observed from Layari River outfall zone to Manora Lighthouse. At Manora Lighthouse, the ratio of freshwater in seawater is substantially low (3–5%). Along southeast coast, mixing of freshwater with seawater is considerably high at the Ghizri area and Ibrahim Haideri Fish Harbor during low tide environment. At sampling stations (Marina Plaza, Casino, and Naval Jetty), the ratio of freshwater into seawater was not significant showing that Malir river water is diluted quickly by strong open sea currents.
Conclusion
The present study has demonstrated that stable isotopes (δ13C, δ15N, δ34S, δ2H, and δ18O) can be effectively used to monitor the marine pollution and to investigate origin of salinity in the coastal aquifer. This study may also provide a precise and accurate isotopic database for researchers interested in seawater pollution and its effect on benthic life.