What are carbon nanotubes?
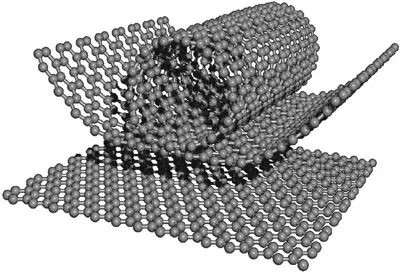
Carbon nanotubes (CNTs) are cylindrical molecules that consist of rolled-up sheets of single-layer carbon atoms (graphene). They can be single-walled (SWCNT) with a diameter of less than 1 nanometer (nm) or multi-walled (MWCNT), consisting of several concentrically interlinked nanotubes, with diameters reaching more than 100 nm. Their length can reach several micrometers or even millimeters.
Like their building block graphene, CNTs are chemically bonded with sp2 bonds, an extremely strong form of molecular interaction. This feature combined with carbon nanotubes’ natural inclination to rope together via van der Waals forces, provide the opportunity to develop ultra-high strength, low-weight materials that possess highly conductive electrical and thermal properties. This makes them highly attractive for numerous applications.

Schematic of how graphene could roll up to form a carbon nanotube.
The rolling-up direction (rolling-up or chiral vector) of the graphene layers determines the electrical properties of the nanotubes. Chirality describes the angle of the nanotube's hexagonal carbon-atom lattice.
Armchair nanotubes – so called because of the armchair-like shape of their edges – have identical chiral indices and are highly desired for their perfect conductivity. They are unlike zigzag nanotubes, which may be semiconductors. Turning a graphene sheet a mere 30 degrees will change the nanotube it forms from armchair to zigzag or vice versa.
While MWCNTs are always conducting and achieve at least the same level of conductivity as metals, SWCNTs' conductivity depends on their chiral vector: they can behave like a metal and be electrically conducting; display the properties of a semi-conductor; or be non-conducting. For example, a slight change in the pitch of the helicity can transform the tube from a metal into a large-gap semiconductor.
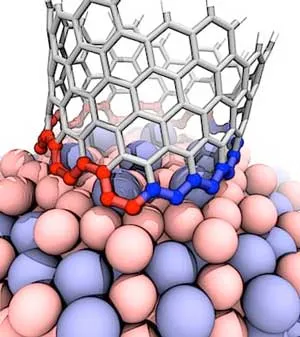
This illustration shows the interface between a growing carbon nanotube and a cobalt-tungsten catalyst. The atomic arrangement of the catalyst forces the nanotube to quickly transition from zigzag (blue) to armchair (red), which ultimately grows a nanotube . (Image: Evgeni Penev/Rice University)
Apart from their electrical properties, which they inherit from graphene, CNTs also have unique thermal and mechanical properties that make them intriguing for the development of new materials:
· their mechanical tensile strength can be 400 times that of steel;
· they are very light-weight – their density is one sixth of that of steel;
· their thermal conductivity is better than that of diamond;
· they have a very high aspect ratio greater than 1000, i.e. in relation to their length they are extremely thin;
· a tip-surface area near the theoretical limit (the smaller the tip-surface area, the more concentrated the electric field, and the greater the field enhancement factor);
· just like graphite, they are highly chemically stable and resist virtually any chemical impact unless they are simultaneously exposed to high temperatures and oxygen - a property that makes them extremely resistant to corrosion;
· their hollow interior can be filled with various nanomaterials, separating and shielding them from the surrounding environment - a property that is extremely useful for nanomedicine applications like drug delivery.
All these properties make carbon nanotubes ideal candidates for electronic devices, chemical/electrochemical and biosensors, transistors, electron field emitters, lithium-ion batteries, white light sources, hydrogen storage cells, cathode ray tubes (CRTs), electrostatic discharge (ESD) and electrical-shielding applications.
Please note that carbon nanotubes are different than carbon nanofibers (CNFs). CNFs are usually several micrometers long and have a diameter of about 200 nm. Carbon fibers have been used for decades to strengthen compound but they do not have the same lattice structure as CNTs. Instead, they consist of a combination of several forms of carbon and/or several layers of graphite, which are stacked at various angles on amorphous carbon (where atoms do not arrange themselves in ordered structures). CNFs have similar properties as CNTs, but their tensile strength is lower owing to their variable structure and they are not hollow inside.
Who discovered carbon nanotubes?
Thousands of papers are being published every year on CNTs or related areas and most of these papers give credit for the discovery of CNTs to Sumio Iijima who, in 1991, published a ground-breaking paper in Nature ("Helical microtubules of graphitic carbon") reporting the discovery of multi-walled carbon nanotubes.
On taking a cursory look at the scientific literature, one might get the impression that Iijima is the de facto discoverer of carbon nanotubes. Of course, there is no doubt that he has made two seminal contributions to the field, however a careful analysis of the literature suggests that certainly he is not the first one who has reported the existence of CNTs.
An editorial in the journal Carbon ("Who should be given the credit for the discovery of carbon nanotubes?") tried to clear the air by describing the chronological events that led to the discovery of carbon nanotubes. By delving deeper into the history of carbon nanotubes, it becomes quite apparent that the origin of CNTs could be even pre-historic in nature (read more here in our article on the birth and early history of carbon nanotubes.

CNT footprints in nature and their respective year of discovery (inset). (click on image to enlarge)
How are carbon nanotubes made?
Three main methods are currently available for the production of CNTs: arc discharge, laser ablation of graphite, and chemical vapor deposition (CVD).
In the first two processes, graphite is combusted electrically or by means of a laser, and the CNTs developing in the gaseous phase are separated. All three methods require the use of metals (e.g. iron, cobalt, nickel) as catalysts.
CVD process
The CVD process currently holds the greatest promise, since it allows the production of larger quantities of CNTs under more easily controllable conditions and at lower cost. In the CVD process, manufacturers can combine a metal catalyst (such as iron) with carbon-containing reaction gases (such as hydrogen or carbon monoxide) to form carbon nanotubes on the catalyst inside a high-temperature furnace.
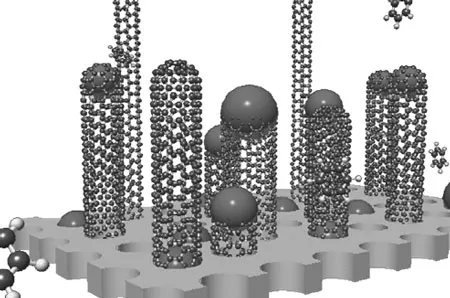
Schematic view of CNT growth on catalyst particles during CVD. First, small secondary catalyst particles of the size of a CNT diameter develop, on which the nanotubes start growing. The catalyst particle is either at the top or at the bottom of the emerging nanotube. Growth will stop if the catalyst particle is deactivated through the development of a carbon envelope. (Image: © Royal Society of Chemistry)
The CVD process can be purely catalytic or plasma-supported. The latter requires slightly lower temperatures (200-500°C) than the catalytic process (up to 750°C) and aims at producing 'lawn-like' CNT growth.
Purification
Even though synthetic techniques have been improved to obtain high-purity carbon nanotubes, the formation of byproducts containing impurities such as metal encapsulated nanoparticles, metal particles in the tip of a carbon nanotube, and amorphous carbon has been an unavoidable phenomenon, because the metal nanoparticles are essential for the nanotube growth.
These foreign nanoparticles, as well as structural defects that occurred during synthesis, have the unfortunate implication that they modify the physico-chemical properties of the produced carbon nanotubes.
That's why carbon nanotubes need to be purified with the help of various methods such as acid treatment or ultrasound at the end of the production process.
Carbon nanotube uses and applications
CNTs are well-suited for virtually any application requiring high strength, durability, electrical conductivity, thermal conductivity and lightweight properties compared to conventional materials.
Currently, CNTs are mainly used as additives to synthetics. CNTs are commercially available as a powder, i.e. in a highly tangled-up and agglomerated form. For CNTs to unfold their particular properties they need to be untangled and spread evenly in the substrate.
Another requirement is that CNTs need to be chemically bonded with the substrate, e.g. a plastic material. For that purpose, CNTs are functionalized, i.e. their surface is chemically adapted for optimal incorporation into different materials and for the specific application in question.
Carbon nanotubes can also be spun into fibers, which not only promise interesting possibilities for specialty textiles but may also help realize a particularly utopian project – the space elevator.
Materials
Carbon nanotube enabled nanocomposites have received much attention as a highly attractive alternative to conventional composite materials due to their mechanical, electrical, thermal, barrier and chemical properties such as electrical conductivity, increased tensile strength, improved heat deflection temperature, or flame retardancy.
These materials promise to offer increased wear resistance and breaking strength, antistatic properties as well as weight reduction. For instance, it has been estimated that advanced CNT composites could reduce the weight of aircraft and spacecraft by up to 30%.
These composite materials already find use in
· sporting goods(bicycle frames, tennis rackets, hockey sticks, golf clubs and balls, skis, kayaks; sports arrows)
· yachting (masts, hulls and other parts of sailboats)
· textiles (antistatic and electrically conducting textiles ('smart textiles'); bullet-proof vests, water-resistant and flame-retardant textiles)
· automotive, aeronautics and space (light-weight, high-strength structural composites)
· industrial engineering (e.g. coating of wind-turbine rotor blades, industrial robot arms)
· electrostatic charge protection (for instance, researchers have a developed electrically conducting and flexible CNT film specifically for space applications) and radiation shielding with CNT-based nanofoams and aerogels.

CNT fabric stopped a 9MM, jacketed round in controlled ballistics testing. This material shown is roughly the same thickness as six stacked business cards. (Source: Nanocomp Technologies)
Catalysis
What makes carbon nanotubes so attractive for catalysis is their exceptionally high surface area combined with the ability to attach essentially any chemical species to their sidewalls. Already, CNTs have been used as catalysts in many relevant chemical processes, however, controlling their catalytic activity is not easy.
Initially, carbon nanotubes have been combined with molecules via very strong bonds (covalent bonds) that lead to very stable compounds. Such connection, however, implies a change in the structure of the nanotube and therefore in its properties.
It would be analogous to nailing an advertisement to a post using a thumbtack: the union is strong, but it leaves a hole in both the advertisement and the post. Weak non-covalent forces have also been used, which keep the structure of the nanotubes intact, but typically yield kinetically unstable compounds. The comparison in this case would be to tape the advert to the post. Neither the advertisement nor the post is damaged, but the union is much weaker.
To overcome this issue, researchers already are developing methods for the chemical modification of carbon nanotubes by mechanical bonding, the first example of mechanically interlocked carbon nanotubes (MINTs). This type of compounds is as stable as covalent compounds, but at the same time as respectful of the initial structure as the non-covalent compounds.
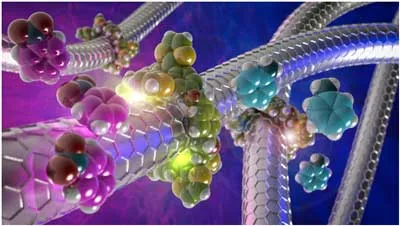
Positive and negative regulation of carbon nanotube catalysts through encapsulation within macrocycles. (Image: Emilio M. Pérez, IMDEA)
Transistors
Despite the rise of graphene and other two-dimensional (2D) materials, semiconducting single-walled carbon nanotubes are still regarded as strong candidates for the next generation of high-performance, ultra-scaled and thin-film transistors as well as for opto-electronic devices to replace silicon electronics (read more: "20 years of nanotube transistors").
One of the crucial questions is if CNT transistors can offer performance advantages over silicon at sub-10 nm lengths.
There have been mixed opinions in the nanoelectronics community regarding whether or not CNT transistors would maintain their impressive performance at extremely scaled lengths. Some argued that the very small effective mass of the carriers would contribute to a tunneling phenomena that would cause the devices to breakdown around 15 nm – an opinion supported by the few theoretical studies that explored nanotube devices at such dimensions.
Meanwhile, others remained convinced that the ultrathin body of single-walled carbon nanotubes – only 1 nm in diameter – would allow for excellent transistor behavior even down to the sub-10 nm range.

Schematic of a sub-10 nm carbon nanotube transistor configuration. (Image: Aaron Scott, Southern Illinois University)
So far researchers have achieved only promising experimental results and at this point there remain numerous challenges related to integrating CNT transistors into industrial-scale chip manufacturing.
Sensors
The group of Cees Dekker paved the way for the development of CNT-based electrochemical nanosensors by demonstrating the possibilities of SWCNTs as quantum wires and their effectiveness in the development of field-effect transistors.
Many studies have shown that although CNTs are robust and inert structures, their electrical properties are extremely sensitive to the effects of charge transfer and chemical doping by various molecules.
Most sensors based on CNTs are field effect transistors (FET) – although CNT are robust and inert structures, their electrical properties are extremely sensitive to the effects of charge transfer and chemical doping by various molecules. CNTs-FETs have been widely used to detect gases such as greenhouse gases in environmental applications.
The functionalization of CNTs is important for making them selective to the target analyte. Different types of sensors are based based on molecular recognition interactions between functionalism CNT and target analytes.
For instance, researchers have developed flexible hydrogen sensors using single-walled carbon nanotubes decorated with palladium nanoparticles.
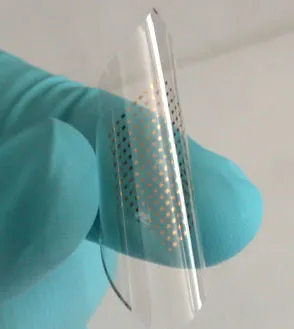
Example of a flexible hydrogen sensor fabricated with single-walled carbon nanotubes. (Image: Dr. Sun/Argonne)
Nano inks
Ink formulations based on CNT dispersions are attractive for printed electronics applications such as transparent electrodes, RFID tags, thin-film transistors, light-emitting devices, and solar cells (read more: "Conductive nanomaterials for printed electronics applications").
Electrodes
Carbon nanotubes have been widely used as electrodes for chemical and biological sensing applications and many other electrochemical studies. With their unique one-dimensional molecular geometry of a large surface area coupled with their excellent electrical properties, CNTs have become important materials for the molecular engineering of electrode surfaces where the development of electrochemical devices with region-specific electron-transfer capabilities is of paramount importance.
Displays
Given their high electrical conductivity, and the incredible sharpness of their tip (the smaller the tips' radius of curvature, the more concentrated the electric field, the higher field emission), carbon nanotubes are considered the most promising material for field emitters and a practical example are CNTs as electron emitters for field emission displays (FED).
Field emission display (FED) technology makes possible a new class of large area, high resolution, low cost flat panel displays. However, FED manufacturing requires CNT to be grown in precise sizes and densities. Height, diameter and tip sharpness affect voltage, while density affects current.
Buckypapers
Buckypapers could find numerous applications: As one of the most thermally conductive materials known, buckypaper could lead to the development of more efficient heat sinks for chips; a more energy-efficient and lighter background illumination material for displays; a protective material for electronic circuits from electromagnetic interference due to its unusually high current-carrying capacity; or switchable surfaces (see: "Nanotechnology paper for switchable surfaces").
Optoelectronic and photonic applications
While individual nanotubes generate discrete fine peaks in optical absorption and emission, macroscopic structures consisting of many CNTs gathered together also demonstrate interesting optical behavior.
For example, a millimeter-long bundle of aligned MWCNTs emits polarized incandescent light by electrical current heating and SWCNT bundles are giving higher brightness emission at lower voltage compared with conventional tungsten filaments.
Nanomedicine and biotechnology
Carbon nanomaterials such as nanotubes or graphene not only are widely researched for their potential uses in industrial applications, they also are of great interest to biomedical engineers working on nanotechnology applications.
There is considerable interest in using CNTs for various biomedical applications. The physical properties of CNTs, such as mechanical strength, electrical conductivity, and optical properties, could be of great value for creating advanced biomaterials.
Carbon nanotubes can also be chemically modified to present specific moieties (e.g., functional groups, molecules, and polymers) to impart properties suited for biological applications, such as increased solubility and biocompatibility, enhanced material compatibility and cellular responsiveness.
Nitrogen-doped carbon nanotubes for instance have been developed for drug delivery applications ("Nanoparticle-corked carbon nanotubes as drug delivery vehicles").
However, the issue of cytotoxicity of CNTs is an area that has already attracted much research interest and has not resulted in a definitive answer yet. Given the inconclusive state of these nanotoxicology studies researchers says that more systematic biological evaluations of CNTs having various chemical and physical properties are warranted in order to determine their precise pharmacokinetics, cytotoxicity, and optimal dosages.
Filtration
High-flow membranes are an important part of future energy-efficient water purification. Already, researchers have demonstrated efficient water transport in carbon nanotubes with openings of less than one nanometer.
When embedded in fatty membranes, the nanotubes squeeze entering water molecules into a single file chain, which leads to very fast transport. The flow was 10 times faster than in wider carbon nanotubes and 6 times faster than in the best biological membrane, a protein called aquaporin (read more: "Filtering water better than nature").
Carbon nanotubes also have been used to demonstrate protective textiles with ultra breathable membranes. These membranes provide rates of water vapor transport that surpass those of commercial breathable fabrics like GoreTex, even though the CNT pores are only a few nanometers wide.
Crucially, they also provide protection from biological agents due to their very small pore size, less than 5 nanometers wide. Biological threats like bacteria or viruses are much larger and typically more than 10-nm in size.