What Is Ion Exchange?
Ion exchange is a water treatment process commonly used for water softening or demineralization, but it also is used to remove other substances from the water in processes such as dealkalization, deionization, and disinfection.
But what exactly is it?
Ion exchange describes a specific chemical process in which unwanted dissolved ions are exchanged for other ions with a similar charge.
Ions are atoms or molecules containing a total number of electrons that are not equal to the total number of protons.
There are two different groups of ions, cations, which are positively charged, and anions, which are negatively charged. We have Michael Faraday to thank for these names, which he devised based on the cation’s attraction to the cathode and the anion’s attraction to the anode in a galvanic device.

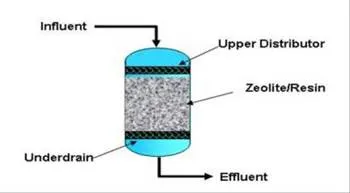
A typical ion exchange configuration.
This attraction is used to remove dissolved ionic contaminants from water. The exchange process occurs between a solid (resin or a zeolite) and a liquid (water). In the process, the less desired compounds are swapped for those that are considered more desirable. These desirable ions are loaded onto the resin material.
In the exchange of cations during water treatment, positively charged ions that come into contact with the ion exchange resin are exchanged with positively charged ions available on the resin surface, usually sodium.
In the anion exchange process, negatively charged ions are exchanged with negatively charged ions on the resin surface, usually chloride. Various contaminants — including nitrate, fluoride, sulfate, and arsenic — can all be removed by anion exchange.
These resins can be used alone or in concert to remove ionic contaminants from the water.
If a substance is not ionic, such as benzene, it cannot be removed via ion exchange.
Recently ion exchange resins have been increasingly used to create drinking water. Specialized resins have been designed to treat various contaminants of concern, including perchlorate and uranium.
There are many resins designed for these purposes, such as strong base/strong anion resin, which is used to remove nitrates and perchlorate. There are also resin beads that can be used for water softening.
Resin materials have a finite exchange capacity. Each of the individual exchange sites will become full with prolonged use. When unable to exchange ions any longer, the resin must be recharged or regenerated to restore it to its initial condition. The substances used for this can include sodium chloride, as well as hydrochloric acid, sulfuric acid, or sodium hydroxide.
The spent regenerant is the primary substance remaining from the process. It contains not only all of the ions removed, but also any extra regenerant ions and will also have a high level of total dissolved solids. It can be treated in a municipal wastewater facility, but discharges may require monitoring.
The efficacy of ion exchange for water treatment can be limited by mineral scaling, surface clogging, and other issues that contribute to resin fouling. Pretreatment processes such as filtration or addition of chemicals can help reduce or prevent these issues.