What is Reverse Osmosis?
To understand the purpose and process of Reverse Osmosis you must first understand the naturally occurring process of Osmosis.
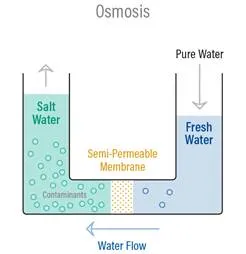
Osmosis is a naturally occurring phenomenon and one of the most important processes in nature. It is a process where a weaker saline solution will tend to migrate to a strong saline solution. Examples of osmosis are when plant roots absorb water from the soil and our kidneys absorb water from our blood.
Below is a diagram which shows how osmosis works. A solution that is less concentrated will have a natural tendency to migrate to a solution with a higher concentration. For example, if you had a container full of water with a low salt concentration and another container full of water with a high salt concentration and they were separated by a semi-permeable membrane, then the water with the lower salt concentration would begin to migrate towards the water container with the higher salt concentration.

A semi-permeable membrane is a membrane that will allow some atoms or molecules to pass but not others. A simple example is a screen door. It allows air molecules to pass through but not pests or anything larger than the holes in the screen door. Another example is Gore-tex clothing fabric that contains an extremely thin plastic film into which billions of small pores have been cut. The pores are big enough to let water vapor through, but small enough to prevent liquid water from passing.
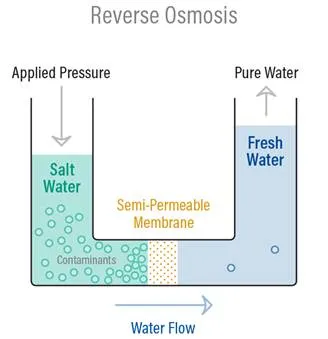
Reverse Osmosis is the process of Osmosis in reverse. Whereas Osmosis occurs naturally without energy required, to reverse the process of osmosis you need to apply energy to the more saline solution. A reverse osmosis membrane is a semi-permeable membrane that allows the passage of water molecules but not the majority of dissolved salts, organics, bacteria and pyrogens. However, you need to 'push' the water through the reverse osmosis membrane by applying pressure that is greater than the naturally occurring osmotic pressure in order to desalinate (demineralize or deionize) water in the process, allowing pure water through while holding back a majority of contaminants.
Below is a diagram outlining the process of Reverse Osmosis. When pressure is applied to the concentrated solution, the water molecules are forced through the semi-permeable membrane and the contaminants are not allowed through.
Reverse Osmosis works by using a high pressure pump to increase the pressure on the salt side of the RO and force the water across the semi-permeable RO membrane, leaving almost all (around 95% to 99%) of dissolved salts behind in the reject stream. The amount of pressure required depends on the salt concentration of the feed water. The more concentrated the feed water, the more pressure is required to overcome the osmotic pressure.
The desalinated water that is demineralized or deionized, is called permeate (or product) water. The water stream that carries the concentrated contaminants that did not pass through the RO membrane is called the reject (or concentrate) stream.
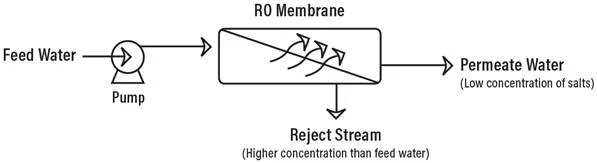
As the feed water enters the RO membrane under pressure (enough pressure to overcome osmotic pressure) the water molecules pass through the semi-permeable membrane and the salts and other contaminants are not allowed to pass and are discharged through the reject stream (also known as the concentrate or brine stream), which goes to drain or can be fed back into the feed water supply in some circumstances to be recycled through the RO system to save water. The water that makes it through the RO membrane is called permeate or product water and usually has around 95% to 99% of the dissolved salts removed from it.
It is important to understand that an RO system employs cross filtration rather than standard filtration where the contaminants are collected within the filter media. With cross filtration, the solution passes through the filter, or crosses the filter, with two outlets: the filtered water goes one way and the contaminated water goes another way. To avoid build up of contaminants, cross flow filtration allows water to sweep away contaminant build up and also allow enough turbulence to keep the membrane surface clean.
Reverse Osmosis is capable of removing up to 99%+ of the dissolved salts (ions), particles, colloids, organics, bacteria and pyrogens from the feed water (although an RO system should not be relied upon to remove 100% of bacteria and viruses). An RO membrane rejects contaminants based on their size and charge. Any contaminant that has a molecular weight greater than 200 is likely rejected by a properly running RO system (for comparison a water molecule has a MW of 18). Likewise, the greater the ionic charge of the contaminant, the more likely it will be unable to pass through the RO membrane. For example, a sodium ion has only one charge (monovalent) and is not rejected by the RO membrane as well as calcium for example, which has two charges. Likewise, this is why an RO system does not remove gases such as CO2 very well because they are not highly ionized (charged) while in solution and have a very low molecular weight. Because an RO system does not remove gases, the permeate water can have a slightly lower than normal pH level depending on CO2 levels in the feed water as the CO2 is converted to carbonic acid.
Reverse Osmosis is very effective in treating brackish, surface and ground water for both large and small flows applications. Some examples of industries that use RO water include pharmaceutical, boiler feed water, food and beverage, metal finishing and semiconductor manufacturing to name a few.
There are a handful of calculations that are used to judge the performance of an RO system and also for design considerations. An RO system has instrumentation that displays quality, flow, pressure and sometimes other data like temperature or hours of operation. In order to accurately measure the performance of an RO system you need the following operation parameters at a minimum:
· Feed pressure
· Permeate pressure
· Concentrate pressure
· Feed conductivity
· Permeate conductivity
· Feed flow
· Permeate flow
· Temperature
This equation tells you how effective the RO membranes are removing contaminants. It does not tell you how each individual membrane is performing, but rather how the system overall on average is performing. A well-designed RO system with properly functioning RO membranes will reject 95% to 99% of most feed water contaminants (that are of a certain size and charge). You can determine how effective the RO membranes are removing contaminants by using the following equation:
Salt Rejection % = | Conductivity of Feed Water – Conductivity of Permeate Water | × 100 |
Conductivity of Feed |
The higher the salt rejection, the better the system is performing. A low salt rejection can mean that the membranes require cleaning or replacement.
This is simply the inverse of salt rejection described in the previous equation. This is the amount of salts expressed as a percentage that are passing through the RO system. The lower the salt passage, the better the system is performing. A high salt passage can mean that the membranes require cleaning or replacement.
Salt Passage % = (1 – Salt Rejection %) |
Percent Recovery is the amount of water that is being 'recovered' as good permeate water. Another way to think of Percent Recovery is the amount of water that is not sent to drain as concentrate, but rather collected as permeate or product water. The higher the recovery % means that you are sending less water to drain as concentrate and saving more permeate water. However, if the recovery % is too high for the RO design then it can lead to larger problems due to scaling and fouling. The % Recovery for an RO system is established with the help of design software taking into consideration numerous factors such as feed water chemistry and RO pre-treatment before the RO system. Therefore, the proper % Recovery at which an RO should operate at depends on what it was designed for. By calculating the % Recovery you can quickly determine if the system is operating outside of the intended design. The calculation for % Recovery is below:
% Recovery = | Permeate Flow Rate (gpm) | × 100 |
Feed Flow Rate (gpm) |
For example, if the recovery rate is 75% then this means that for every 100 gallons of feed water that enter the RO system, you are recovering 75 gallons as usable permeate water and 25 gallons are going to drain as concentrate. Industrial RO systems typically run anywhere from 50% to 85% recovery depending the feed water characteristics and other design considerations.
The concentration factor is related to the RO system recovery and is an important equation for RO system design. The more water you recover as permeate (the higher the % recovery), the more concentrated salts and contaminants you collect in the concentrate stream. This can lead to higher potential for scaling on the surface of the RO membrane when the concentration factor is too high for the system design and feed water composition.
Concentration Factor = | 1 |
1 – Recovery % |
The concept is no different than that of a boiler or cooling tower. They both have purified water exiting the system (steam) and end up leaving a concentrated solution behind. As the degree of concentration increases, the solubility limits may be exceeded and precipitate on the surface of the equipment as scale.
For example, if your feed flow is 100 gpm and your permeate flow is 75 gpm, then the recovery is (75/100) x 100 = 75%. To find the concentration factor, the formula would be 1 ÷ (1-75%) = 4.
A concentration factor of 4 means that the water going to the concentrate stream will be 4 times more concentrated than the feed water is. If the feed water in this example was 500 ppm, then the concentrate stream would be 500 x 4 = 2,000 ppm.
Gfd = | gpm of permeate × 1,440 min/day |
# of RO elements in system × square footage of each RO element |
For example, you have the following:
The RO system is producing 75 gallons per minute (gpm) of permeate. You have 3 RO vessels and each vessel holds 6 RO membranes. Therefore you have a total of 3 x 6 = 18 membranes. The type of membrane you have in the RO system is a Dow Filmtec BW30-365. This type of RO membrane (or element) has 365 square feet of surface area.
To find the flux (Gfd):
Gfd = | 75 gpm × 1,440 min/day | = | 108,000 |
18 elements × 365 sq ft | 6,570 |
The flux is 16 Gfd.
This means that 16 gallons of water is passed through each square foot of each RO membrane per day. This number could be good or bad depending on the type of feed water chemistry and system design. Below is a general rule of thumb for flux ranges for different source waters and can be better determined with the help of RO design software. If you had used Dow Filmtec LE-440i RO membranes in the above example, then the flux would have been 14. So it is important to factor in what type of membrane is used and to try and keep the type of membrane consistent throughout the system.
Feed Water Source | Gfd |
Sewage Effluent | 5-10 |
Sea Water | 8-12 |
Brackish Surface Water | 10-14 |
Brackish Well Water | 14-18 |
RO Permeate Water | 20-30 |
A Mass Balance equation is used to help determine if your flow and quality instrumentation is reading properly or requires calibration. If your instrumentation is not reading correctly, then the performance data trending that you are collecting is useless. You will need to collect the following data from an RO system to perform a Mass Balance calculation:
1. Feed Flow (gpm)
2. Permeate Flow (gpm)
3. Concentrate Flow (gpm)
4. Feed Conductivity (µS)
5. Permeate Conductivity (µS)
6. Concentrate Conductivity (µS)
The mass balance equation is:
(Feed flow1 x Feed Conductivity) = (Permeate Flow x Permeate Conductivity)
+ (Concentrate Flow x Concentrate Conductivity)
1Feed Flow equals Permeate Flow + Concentrate Flow
For example, if you collected the following data from an RO system:
Permeate Flow | 5 gpm |
Feed Conductivity | 500 µS |
Permeate Conductivity | 10 µS |
Concentrate Flow | 2 gpm |
Concentrate Conductivity | 1200 µS |
Then the Mass Balance Equation would be:
(7 x 500) = (5 x 10) + (2 x 1200)
3,500 ≠ 2,450
Then find the difference
(Difference / Sum) x 100
((3,500 - 2,450) / (3,500 + 2,450)) x 100
= 18%
A difference of +/- 5% is ok. A difference of +/- 5% to 10% is generally adequate. A difference of > +/- 10% is unacceptable and calibration of the RO instrumentation is required to ensure that you are collecting useful data. In the example above, the RO mass balance equation falls out of range and requires attention.