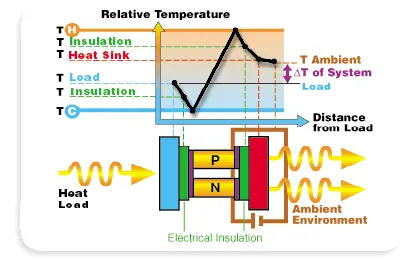
Thermoelectric Principle
Thermoelectricity means the direct conversion of heat into electric energy, or vice versa. The term is generally restricted to the irreversible conversion of electricity into heat described by the English physicist James P. Joule and to three reversible effects named for Seebeck, Peltier, and Thomson, their respective discoverers.
According to Joule's law, a conductor carrying a current generates heat at a rate proportional to the product of the resistance (R) of the conductor and the square of the current (I). The German physicist Thomas J. Seebeck discovered in the 1820s that if a closed loop is formed by joining the ends of two strips of dissimilar metals and the two junctions of the metals are at different temperatures, an electromotive force, or voltage, arises that is proportional to the temperature difference between the junctions. A circuit of this type is called a thermocouple; a number of thermocouples connected in series is called a thermopile.
In 1834 the French physicist Jean C. A. Peltier discovered an effect inverse to the Seebeck effect: If a current passes through a thermocouple, the temperature of one junction increases and the temperature of the other decreases, so that heat is transferred from one junction to the other. The rate of heat transfer is proportional to the current and the direction of transfer is reversed if the current is reversed.
Thermoelectric materials are of interest for applications as heat pumps and power generators. The performance of thermoelectric devices is quantified by a figure of merit, ZT, where Z is a measure of a material's thermoelectric properties and T is the absolute temperature. A material with a figure of merit of around unity was first reported over four decades ago, but until recently, there has been only modest progress in finding materials with enhanced ZT values at room temperature.
