VISCOSITY
Viscosity is defined as the property of a fluid which offers resistance to the
movement of one layer of fluid over adjacent layer of the fluid. When two layers of a fluid, a distance ‘dy’ apart, move one over the other at different velocities, say u
and u+du as shown in figure. The viscosity together with relative velocity causes a shear stress acting between the fluid layers
The top layer causes a shear stress on the adjacent lower layer while the lower layer causes a shear stress on the adjacent top layer. This shear stress is proportional to the rate of change of velocity with respect to y.
VAPOUR PRESSURE
The pressure at which a liquid will boil is called its vapor pressure. This pressure is a function 3 of temperature (vapor pressure increases with temperature). In this context we usually think about the temperature at which boiling occurs. For example, water boils at 100oC at sea-level atmospheric pressure (1 atm abs). However, in terms of vapor pressure, we can say that by increasing the temperature of water at sea level to 100 oC, we increase the vapor pressure to the point at which it is equal to the atmospheric pressure (1 atm abs), so that boiling occurs. It is easy to visualize that boiling can also occur in water at temperatures much below 100oC if the pressure in the water is reduced to its vapor pressure. For example, the vapor pressure of water at 10oC is 0.01 atm.
1.CAVITATION
Cavitation(flashing of the liquid into vapour) takes place when very low pressures are produced at certain locations of a flowing liquid. Cavitation results in the formation of vapour pockets or cavities which are carried away from the point of origin and collapse at the high pressure zone.
COMPRESSIBILITY
Compressibility is the reciprocal of the bulk modulus of elasticity, K which is defined as the ratio of compressive stress to volumetric strain.
Compressibility is given by = 1/K
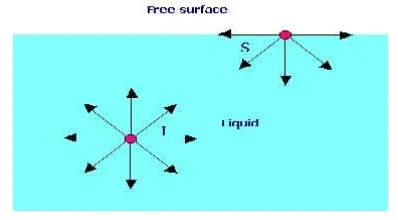
SURFACE TENSION
Surface tension is defined as the tensile force acting on the surface of a liquid in contact with a gas or on the surface between two two immiscible liquids such that the contact surface behaves like a membrane under tension.

1.A soap bubble 50 mm in diameter contains a pressure (in excess of atmospheric) of 2 bar. Find the surface tension in the soap film.
Data:
Radius of soap bubble (r) = 25 mm = 0.025 m Dp = 2 Bar = 2 x 105 N/m2
Formula:
Pressure inside a soap bubble and surface tension (s) are related by, Dp = 4s/r
Calculations:
s = Dpr/4 = 2 x 105 x 0.025/4 = 1250 N/m