IRON CARBON DIAGRAM
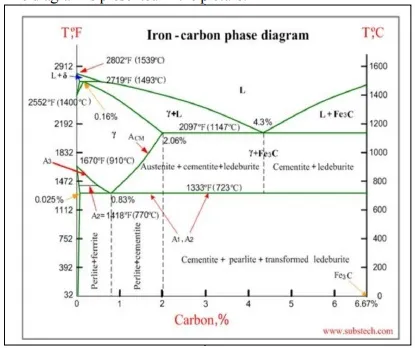
Iron-carbon phase diagram
Iron-carbon phase diagram describes the iron-carbon system of alloys containing up to 6.67% of carbon, discloses the phases compositions and their transformations occurring with the alloys during their cooling or heating. Carbon content 6.67% corresponds to the fixed c omposition of the iron carbide Fe3C.
The diagram is presented in the picture:

The following phases are involved in the transformation, occurring with iron-carbon alloys: L - Liquid solution of carbon in iron;
δ-ferrite - Solid solution of carbon in iron.
Maximum concentration of carbon in δ-ferrite is 0.09% at 2719 ºF (1493ºC) - temperature of the peritectic transformation.
The crystal structure of δ-ferrite is BCC (cubic body centered). Austenite - interstitial solid solution of carbon in γ-iron.
Austenite has FCC (cubic face centered) crystal structure, permitting high solubility of carbon - up to 2.06% at 2097 ºF (1147 ºC).
Austenite does not exist below 1333 ºF (723ºC) and maximum carbo n concentration at this temperature is 0.83%.
α-ferrite - solid solution o f carbon in α-iron. α-ferrite has BCC crystal structure and low solubility of carbon - up to 0.25% at 1333 ºF (723ºC). α-ferrite exists at room temperature.
Cementite - iron carbide , intermetallic compound, having fixed com position Fe3C.
Cementite is a hard and brittle substance, influencing on the properties of steels and cast irons.
The following phase transformations occur with iron-carbon alloys:
Alloys, containing up to 0.51% of carbon, start soli dification with formation of crystals of δ-ferrite. Carbon content in δ-ferrite increases up to 0.09% in course solidification, and at 2719 ºF (1493ºC) remaining liquid phase and δ- ferrite perform peritectic transformation, resulting in formation of austenite.
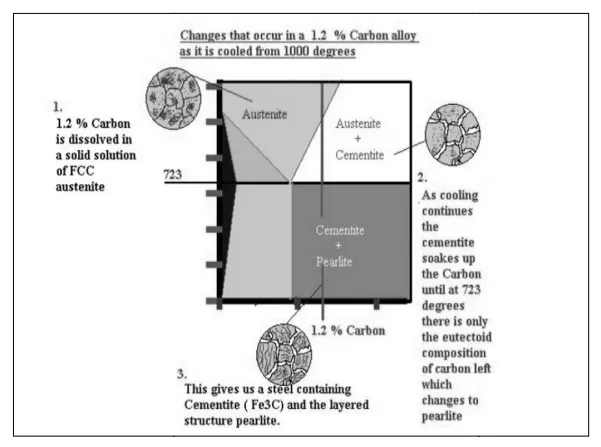
Alloys, containing carbon more than 0.51%, but less than 2.06%, form primary austenite crystals in the beginning of solidification and when the temperature reaches the curve ACM primary cementite stars to form.
Iron-carbon alloys, containing up to 2.06% of carbon, are called steels.
Alloys, containing from 2.06 to 6.67% of carbon, experience eutectic transformation at 2097 ºF (1147 ºC). The eutectic concentration of carbon is 4.3%.
In practice only hypoeutectic alloys are used. These alloys (carbon content from 2.06% to 4.3%) are called cast irons. When temperature of an alloy from this range reaches 2097 ºF (1147 ºC), it contains primary austenite crystals and some amount of the liquid phase. The latter decomposes by eutectic mechanism to a fine mixture of austenite and cementite, called ledeburite.
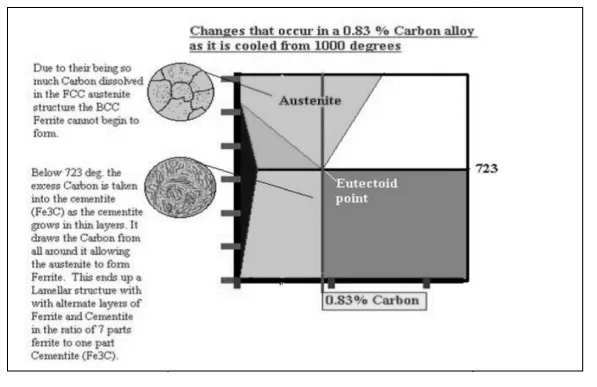
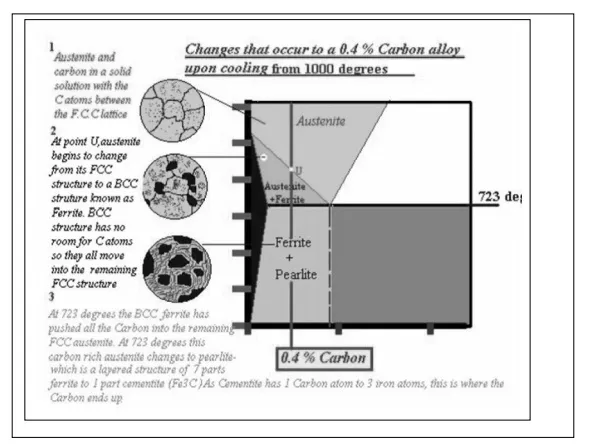
All iron-carbon alloys (steels and cast irons) experience eutectoid transformation at 1333 ºF (723ºC). The eutectoid concentration of carbon is 0.83%.
When the temperature of an alloy reaches 1333 ºF (733ºC), austenite transforms to pearlite (fine ferrite-cementite structure, forming as a result of decomposition of austenite at slow cooling conditions).
Critical temperatures
Upper critical temperature (point) A3 is the temperature, below which ferrite starts to form as a result of ejection from austenite in the hypoeutectoid alloys.
Upper critical temperature (point) ACM is the temperature, below which cementite starts to form as a result of ejection from austenite in the hypereutectoid alloys.
Lower critical temperature (point) A1 is the temperature of the austeniteto-pearlite eutectoid transformation. Below this temperature austenite does not exist.
Magnetic transformation temperature A2 is the temperature below which α-ferrite is ferromagnetic.
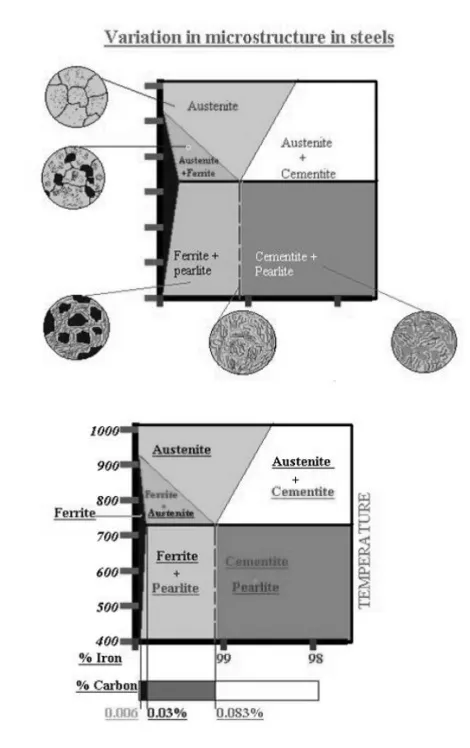
Phase compositions of the iron-carbon alloys at room temperature
o Hypoeutectoid steels (carbon content from 0 to 0.83%) consist of primary (proeutectoid) ferrite (according to the curve A3) and pearlite.
o Eutectoid steel (carbon content 0.83%) entirely consists of pearlite.
o Hypereutectoid steels (carbon content from 0.83 to 2.06%) consist of primary (proeutectoid) cementite (according to the curve ACM) and pearlite.
o Cast irons (carbon content from 2.06% to 4.3%) consist of proeutectoid cementite C2 ejected from austenite according to the curve ACM , pearlite and transformed ledeburite (ledeburite in which austenite transformed to pearlite).
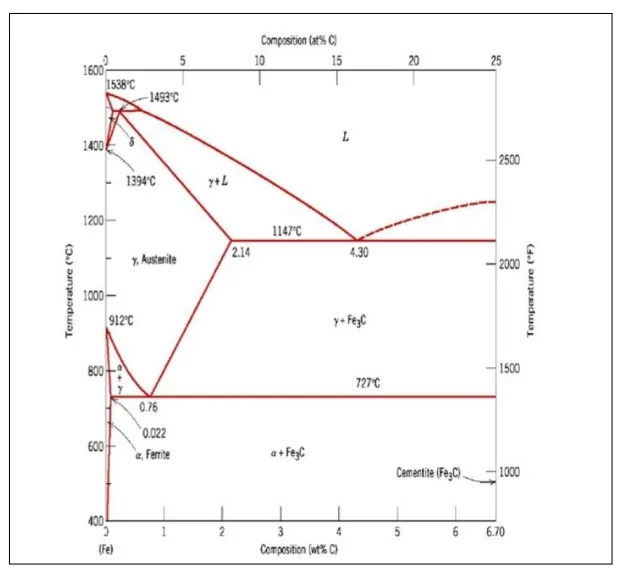
At 4.3% carbon composition, on cooling Liquid phase is converted in to two solids
hence forming Eutectic reaction.
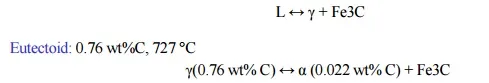
L ↔ γ + Fe3C
Eutectoid: 0.76 wt%C, 727 °C
γ(0.76 wt% C) ↔ α (0.022 wt% C) + Fe3C
Shown below is the steel part of the iron carbon diagram containing up to 2% Carbon. At the eutectoid point 0.83% Carbon, Austenite which is in a solid solution changes directly into a solid known as Pearlite which is a layered structure consisting of layers of Ferrite and Cementite