Thermal Treatments (Heat-Treating)
In the previous pages on the subjects of alloying and the binary phase diagram, the microstructures of alloys that were allowed to solidify by slow cooling were considered. It should also be known, however, that it is possible to modify the microstructure of an alloy by subjecting it to various thermal treatments. Heat-treating is a term used to describe all of the controlled heating and cooling operations performed on a material in the solid state for the purpose of altering its microstructure and/or properties. The focus of this discussion will be on metals but is should be noted that heat-treatment is also used on ceramics and composites to modify their properties.
The major objectives of the different kinds of thermal treatments are:
Different metals respond to treatment at different temperatures. Each metal has a specific chemical composition, so changes in physical and structural properties take place at different, critical temperatures. Even small percentages of elements in the metal composition, such as carbon, will greatly determine the temperature, time, method and rate of cooling that needs to be used in the heat treating process. Depending on the thermal treatment used, the atomic structure and/or microstructure of a material may change due to movement of dislocations, an increase or decrease in solubility of atoms, an increase in grain size, the formation of new grains of the same or different phase, a change in the crystal structure, and others mechanisms.
Since there are so many ways in which metals are heat treated, it is not practical to discuss them all. But, as an example, let’s look at how heat treatment is used to strengthen a copper aluminum alloy.
Precipitation Hardening
In designing alloys for strength, an approach often taken is to develop an alloy with a structure that consists of particles (which impede dislocation movement) dispersed in a ductile matrix. Such a dispersion can be obtained by choosing an alloy that is a single phase at elevated temperature but on cooling will precipitate another phase in the matrix. A thermal process is then developed to produce the desired distribution of precipitate in the matrix. When the alloy is strengthened by this thermal treatment, it is called precipitation strengthening or hardening.
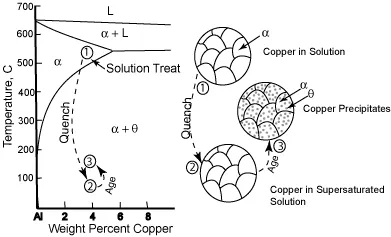
Precipitation hardening consists of three main steps: solution treatment, quenching, and aging. Solution treatment involves heating the alloy to a temperature that allows the alloying atoms (called the solute) to dissolve into the solution. This results in a homogeneous solid solution of one phase. Quenching rapidly cools the solution and freezes the atoms in solution. In more technical terms, the quenching cools the material so fast that the atoms of the alloying elements do not have time to diffuse out of the solution. In the as-quenched condition, the solute is supersaturated meaning that the lattice is overly stressed by the alloying atoms. Aging is the process where the solute particles diffuse out of solution and into clusters that distort and strengthen the material.
The precipitation hardening process for a copper-aluminum alloy is shown graphically in the image below. On the right is phase diagram, which is a very useful tool for understanding and controlling polyphase structures. The phase diagram is simply a map showing the structure of phases present as the temperature and overall composition of the alloy are varied. The images on the right in the image show the resulting microstructure at each step in the process.

Common Heat Treating Processes
A few of the more common terms used in heat treating are introduced below. It should be noted that not all of the term are applicable to all alloys.
Age Hardening is a relatively low-temperature heat treatment process that strengthens a material by causing the precipitation of components or phases of alloy from a super-saturated solid solution condition.
Annealing is a softening process in which metals are heated and then allowed to cool slowly. The purpose of annealing is to soften the material for improve machinability, formability, and sometimes to control magnetic properties.
Normallizing is much like annealing, but the cooling process is much faster. This results in increased strength but less ductility in the metal. Its purpose is to refine grain structure, produce more uniform mechanical properties, and sometimes to relieve internal and surface stresses.
Precipitation Heat Treatment is the three step process of solution treating, quenching, and age hardening to increase the strength or hardness of an alloy.
Solution Heat Treatment involves heating the material to a temperature that puts all the elements in solid solution and then cooling very rapidly to freeze the atoms in place.
Stress Relieving is a low temperature heat treat process that is used to reduce the level of residual stresses in a material.
Tempering involves gently heating a hardened metal and allowing it to cool slowly will produce a metal that is still hard but also less brittle. This process is known as tempering.
Quenching is the rapid cooling of a hot material. The medium used to quench the material can vary from forced air, oil, water and others. Many steels are hardened by heating and quenching. Quenching results in a metal that is very hard but also brittle.