BASIC PRINCIPLES OF HEAT TREATMENT
Heat treatment of a metal or alloy is a technological procedure, including controlled heating and cooling operations, conducted for the purpose of changing the alloy microstructure and resulting in achieving required properties.
There are two general objectives of heat treatment: hardening and annealing.
HARDENING
Hardening is a process of increasing the metal hardness, strength, toughness, fatigue resistance.
· Strain hardening (work hardening) – strengthening by cold work (cold deformation)
Cold plastic deformation causes increase of concentration of dislocations, which mutually entangle one another, making further dislocation motion difficult and therefore resisting the deformation or increasing the metal strength.
· Grain size strengthening (hardening) - strengthening by grain refining.
Grain boundaries serve as barriers to dislocations, raising the stress required to cause plastic deformation.
• Solid solution hardening- strengthening by dissolving an alloying element.
Atoms of solute element distort the crystal lattice, resisting the dislocations motion. Interstitial elements are more effective in solid solution hardening, than substitution elements.
• Dispersion strengthening – strengthening by adding second phase into metal matrix.
The second phase boundaries resist the dislocations motions, increasing the material strength. The strengthening effect may be significant if fine hard particles are added to a soft ductile matrix (composite materials).
Hardening by result of Spinodal decomposition. Spinodal structure is characterized by strains on the coherent boundaries between the Spinodal phases causing hardening of the alloy.

• Precipitation hardening (age hardening) - strengthening by precipitation of fine particles of a second phase from a supersaturated solid solution.
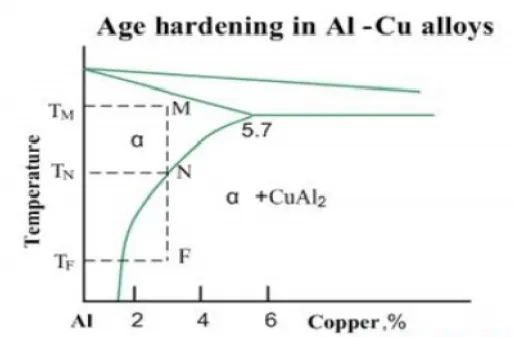
The second phase boundaries resist the dislocations motions, increasing the material strength. The age hardening mechanism in Al-Cu alloys may be illustrated by the phase diagram of Al-Cu system. When an alloy Al-3%Cu is heated up to the temperature TM, all CuAl2 particles are dissolved and the alloy exists in form of single phase solid solution (α-phase). This operation is called solution treatment.
Slow cooling of the alloy will cause formation of relatively coarse particles of CuAl2 intermetallic phase, starting from the temperature TN.However if the the cooling rate is high (quenching), solid solution will retain even at room temperature TF. Solid solution in this non-equilibrium state is called supersaturated solid solution.
Obtaining of supersaturated solid solution is possible when cooling is considerably faster, than diffusion processes. As the diffusion coefficient is strongly dependent on the the precipitation of CuAl2 from supersaturated temperature, solution is much faster at elevated temperatures (lower than TN).This process is called artificial aging. It takes usually a time from several hours to one day. When the aging is conducted at the room temperature, it is called natural aging. Natural aging takes several days or more.
Precipitation from supersaturate d solid solution occurred in several steps:
· Segregation of Cu atoms into plane clusters. These clusters are called called Guinier-Preston1 zones (G-P1 zones).
· Diffusion of Cu atoms to the G -P1 zones and formation larger clusters, called GP2 zones or θ” phase. This phase is coherent with the matrix .
· Formation of ‘θ’ phase which is partially coherent with the matrix. This phase provides maximum hardening.