Transformation rate effects and TTT diagrams, Microstructure and Property Changes in Fe-C Alloys
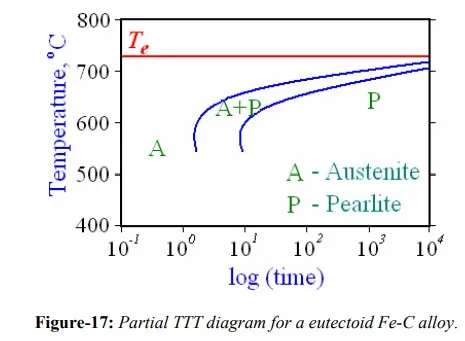
Solid state transformations, which are very important in steels, are known to be dependent on time at a particular temperature, as shown in figure-14(b). Isothermal transformation diagram, also known as TTT diagram, measures the rate of transformation at a constant temperature i.e. it shows time relationships for the phases during isothermal transformation. Information regarding the time to start the transformation and the time required to complete the transformation can be obtained from set of TTT diagrams. One such set of diagram for reaction of austenite to pearlite in steel is shown in figure-17. The diagram is not complete in the sense that the transformations of austenite that occur at temperatures below about 550 ْC are not shown.

As mentioned in previous section, thickness of layers in pearlite depends on the temperature at which the transformation occurred. If the transformation took place at a temperature that is just below the eutectoid temperature, relatively thick layers of α– ferrite and cementite are produced in what is called coarse pearlite. This is because of high diffusion rates of carbon atoms. Thus with decreasing transformation temperature, sluggish movement of carbon results in thinner layers α–ferrite and cementite i.e. fine pearlite is produced.
At transformation temperatures below 550 ْC, austenite results in different product known as bainite. Bainite also consists of α–ferrite and cementite phases i.e. transformation is again diffusion controlled but morphologically it consists of very small particles of cementite within or between fine ferrite plates. Bainite forms needles or plates, depending on the temperature of the transformation; the microstructural details of bainite are so fine that their resolution is only possible using electron microscope. It differs from pearlite in the sense that different mechanism is involved in formation ob bainite which does not have alternating layers of α–ferrite and cementite. In addition, because of equal growth rates in all directions pearlite tends to form spherical colonies, whereas bainite grows as plates and has a characteristic acicular (needlelike) appearance. Upper bainite, formed at the upper end of the temperature range (550 ْ C-350 ْC), is characterized by relatively coarse, irregular shaped cementite particles in α–ferrite plates. If the transformation is taking place at lower temperatures (350 ْ C-250 ْC), the α– ferrite plates assume a more regular needlelike shape, and the transformation product is called lower bainite. At the same time carbide particles become smaller in size and appear as cross-striations making an angle of about 55 ْ to the axis of the α–ferrite plate. Upper bainite has large rod-like cementite regions, whereas lower bainite has much finer cementite particles as a result of sluggish diffusion of carbon atoms at lower temperatures. Lower bainite is considerably harder than upper bainite. Another characteristic of bainite is that as it has crystallographic orientation that is similar to that found in simple ferrite nucleating from austenite, it is believed that bainite is nucleated by the formation of ferrite. This is in contrast to pearlite which is believed to be nucleated by formation of cementite.
Basically, bainite is a transformation product that is not as close to equilibrium as pearlite. The most puzzling feature of the bainite reaction is its dual nature. In a number of respects, it reveals properties that are typical of a nucleation and growth type of transformation such as occurs in the formation pearlite and also a mixture of α–ferrite and cementite though of quite different morphology (no alternate layers), but at the same time it differs from the Martensite as bainite formation is athermal and diffusion controlled though its microstructure is characterized by acicular (needlelike) appearance.
The time-temperature dependence of the bainite transformation can also be presented using TTT diagram. It occurs at temperatures below those at which pearlite forms i.e. it does not form until the transformation temperature falls below a definite temperature, designated as BB s. Above this temperature austenite does not form bainite except under external stresses. Below BsB , austenite does not transform completely to bainite. The amount of bainite formed increases as the isothermal reaction temperature is lowered. By reaching a lower limiting temperature, BB f, it is possible to transform austenite completely to bainite. The BsB and BB f temperatures are equivalent to the Ms and Mf temperatures for Martensite.
In simple eutectoid steels, pearlite and bainite transformations overlap, thus transition from the pearlite to bainite is smooth and continuous i.e. knees of individual pearlite and bainite curves are merged together. However each of the transformations has a characteristic C-curve, which can be distinguishable in presence of alloying elements. As shown in complete TTT diagram for eutectoid steel in figure-18, above approximately 550 ْ C-600 ْC, austenite transforms completely to pearlite. Below this range up to 450 ْC, both pearlite and bainite are formed. Finally, between 450 ْC and 210 ْC, the reaction product is bainite only. Thus bainite transformation is favored at a high degree of supercooling, and the pearlite transformation at a low degree of supercooling. In middle region, pearlitic and bainitic transformations are competitive with each other.
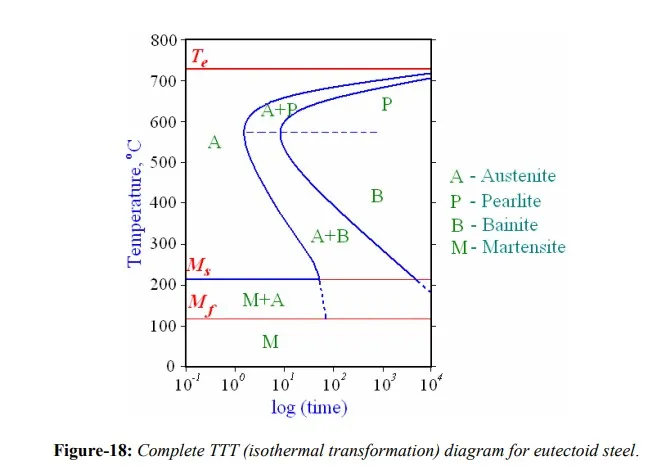
As explained in earlier section, martensitic transformation can dominate the proceedings if steel is cooled rapid enough so that diffusion of carbon can be arrested. Transformation of austenite to Martensite is diffusion-less, time independent and the extent of transformation depends on the transformation temperature. Martensite is a meta-stable phase and decomposes into ferrite and pearlite but this is extremely slow (and not noticeable) at room temperature. Alloying additions retard the formation rate of pearlite and bainite, thus rendering the martensitic transformation more competitive. Start of the transformation is designated by Ms, while the completion is designated by Mf in a transformation diagram. Martensite forms in steels possesses a body centered tetragonal crystal structure with carbon atoms occupying one of the three interstitial sites available. This is the reason for characteristic structure of steel Martensite instead of general BCC. Tetragonal distortion caused by carbon atoms increases with increasing carbon content and so is the hardness of Martensite. Austenite is slightly denser than Martensite, and therefore, during the phase transformation upon quenching, there is a net volume increase. If relatively large pieces are rapidly quenched, they may crack as a result of internal stresses, especially when carbon content is more than about 0.5%.
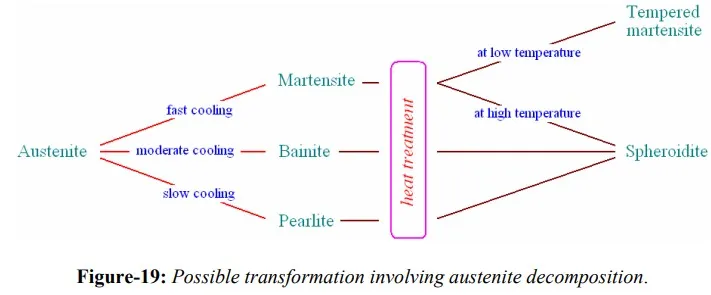
Mechanically, Martensite is extremely hard, thus its applicability is limited by brittleness associated with it. Characteristics of steel Martensite render it unusable for structural applications in the as-quenched form. However, structure and thus the properties can be altered by tempering, heat treatment observed below eutectoid temperature to permit diffusion of carbon atoms for a reasonable period of time. During tempering, carbide particles attain spherical shape and are distributed in ferrite phase – structure called spheroidite. Spheroidite is the softest yet toughest structure that steel may have. At lower tempering temperature, a structure called tempered Martensite forms with similar microstructure as that of spheroidite except that cementite particles are much, much smaller. The tempering heat treatment is also applicable to pearlitic and bainitic structures. This mainly results in improved machinability. The mechanism of tempering appears to be first the precipitation of fine particles of hexagonal ε-carbide of composition about Fe2.4C from Martensite, decreasing its tetragonality. At higher temperatures or with increasing tempering times, precipitation of cementite begins and is accompanied by dissolution of the unstable ε-carbide. Eventually the Martensite loses its tetragonality and becomes BCC ferrite, the cementite coalesces into spheres. A schematic of possible transformations involving austenite decomposition are shown in figure-19.
Tempering of some steels may result in a reduction of toughness what is known as temper embrittlement. This may be avoided by (1) compositional control, and/or (2) tempering above 575 or below 375, followed by quenching to room temperature. The effect is greatest in Martensite structures, less severe in bainitic structures and least severe in pearlite structures. It appears to be associated with the segregation of solute atoms to the grain boundaries lowering the boundary strength. Impurities responsible for temper brittleness are: P, Sn, Sb and As. Si reduces the risk of embrittlement by carbide formation. Mo has a stabilizing effect on carbides and is also used to minimize the risk of temper brittleness in low alloy steels.
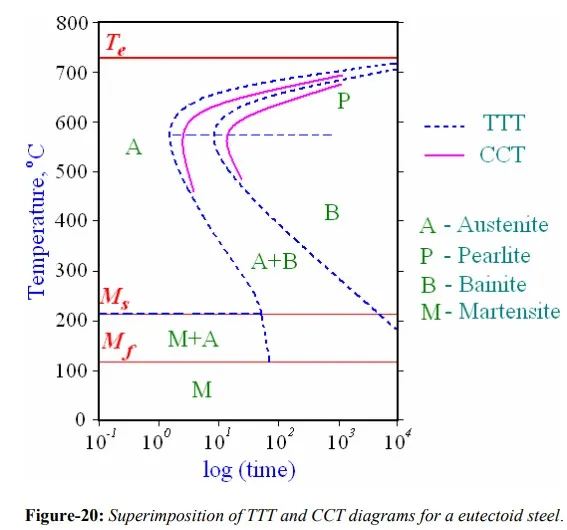
TTT diagrams are less of practical importance since an alloy has to be cooled rapidly and then kept at a temperature to allow for respective transformation to take place. However, most industrial heat treatments involve continuous cooling of a specimen to room temperature. Hence, Continuous Cooling Transformation (CCT) diagrams are generally more appropriate for engineering applications as components are cooled (air cooled, furnace cooled, quenched etc.) from a processing temperature as this is more economic than transferring to a separate furnace for an isothermal treatment. CCT diagrams measure the extent of transformation as a function of time for a continuously decreasing temperature. For continuous cooling, the time required for a reaction to begin and end is delayed, thus the isothermal curves are shifted to longer times and lower temperatures.
Both TTT and CCT diagrams are, in a sense, phase diagrams with added parameter in form of time. Each is experimentally determined for an alloy of specified composition. These diagrams allow prediction of the microstructure after some time period for constant temperature and continuous cooling heat treatments, respectively. Normally, bainite will not form during continuous cooling because all the austenite will have transformed to pearlite by the time the bainite transformation has become possible. Thus, as shown in figure-20, region representing austenite-pearlite transformation terminates just below the nose.