Particle strengthening by precipitation and precipitation reactions
As explained in an earlier chapter (chapter-6: Dislocations and Strengthening Mechanisms), by obstructing dislocation motion in different means, material’s strength can be increased. One of the methods that are applicable to multi-phase material is particle strengthening in which second phase particles are introduced into the matrix by either mixing-and-consolidation (dispersion strengthening) or precipitated in solid state (precipitation hardening).
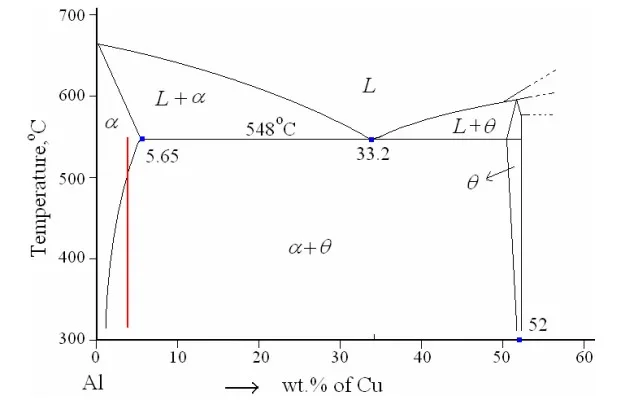
The object of the precipitation strengthening is to create in a heat-treated alloy a dense and fine dispersion of precipitated particles in a matrix of deformable metal. The particles act as obstacles to dislocation motion. In order for an alloy system to be able to precipitation-strengthened for certain alloy compositions; there must be a terminal solid solution which has a decreasing solid solubility as the temperature decreases. For example: Au-Cu in which maximum solid solubility of Cu in Al is 5.65% at 548 ْ C that decreases with decreasing temperature.
The precipitation strengthening process involves the following three basic steps:
v Solutionizing (solution heat treatment), where the alloy is heated to a temperature between solvus and solidus temperatures and kept there till a uniform solidsolution structure is produced.
v Quenching, where the sample is rapidly cooled to a lower temperature (room temperature) and the cooling medium is usually water. Alloy structure in this stage consists of supersaturated solid solution.
v Aging is the last but critical step. During this heat treatment step finely dispersed precipitate particle will form. Aging the alloy at room temperature is called natural aging, whereas at elevated temperatures is called artificial aging. Most alloys require artificial aging, and aging temperature is usually between 15-25% of temperature difference between room temperature and solution heat treatment temperature.
Precipitation strengthening and reactions that occur during precipitation can be best illustrated using the Al-4%Cu (duralumin) system. Figure-10 depicts the Al-rich end of the Al-Cu phase diagram. It can be observed that the alloy with 4%Cu exists as a single phase α-solid solution at around 550 ْ C, and at room temperature as a mixture of α (with less than 0.5%Cu) and an inter-metallic compound, CuAl2 (θ) with 52%Cu. On slow cooling α rejects excess Cu as precipitate particles of θ. These particles relatively coarse in size and can cause only moderate strengthening effect.

Figure-10: Aluminium rich end of Al-Cu phase diagram.
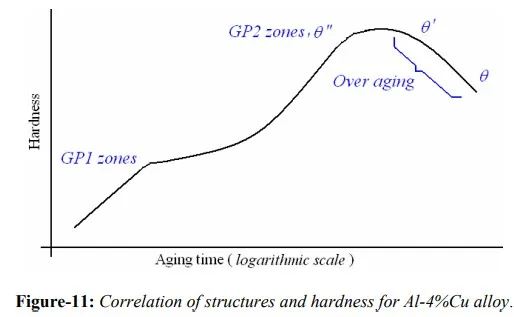
By rapidly cooling the alloy, a supersaturated solution can be obtained at room temperature. As a function of time at room temperature, and at higher temperatures up to 2000c, the diffusion of Cu atoms may take place and the precipitate particles can form. For this particular alloy, Al-4%Cu, five sequential structures can be identified: (a) supersaturated solid solution α, (b) GP1 zones, (c) GP2 zones (θ” phase), (d) θ’ phase and (e) θ phase, CuAl2. Not all these phases can be produced at all aging temperatures. GP1 and GP2 zones are produced at lower temperatures, and θ’ and θ phases occur at higher temperatures. The initial stages of precipitation are the most difficult to analyze because of the extremely small size of the particles and their relatively uniform distribution. GP zones meant for Guinier-Preston zones which have a definite composition and structure that is not the same as that of the final stable precipitate. Evidently these particles are easier to nucleate than the final precipitate, as a result, form first. Eventually they disappear as later more stable phases appear. θ” and θ’ are metastable transition precipitates with distinct crystal structure of their own, while θ is the equilibrium stable precipitate of CuAl2.
GP1 zones:- These zones are created by Cu atoms segregating in α, and the segregated regions are of disk shape with thickness of 0.4-0.6 nm, and 8-10 nm in diameter and form on the {100} cubic planes of the matrix. As Cu atoms which replace Al atoms are smaller in diameter, matrix lattice strains tetragonally. These zones are said to be coherent with the matrix lattice.
GP2 zones / θ” phase:- With additional aging, ordering of larger clumps of Cu atoms on {100} occurs. These zones have tetragonal structure which therefore introduces coherency in the lattice with {100} planes of the matrix, accompanied by further hardening. However, their size ranges from 1-4 nm thick and 10-100 nm in diameter as aging proceeds.
θ’ phase:- This phase nucleates heterogeneously especially on dislocations. It has tetragonal structure but is partially coherent with the matrix. This phase forms platelets with thickness 10-150 nm
θ phase:- With still further aging the equilibrium phase CuAl2 or θ is formed from the transition lattice θ’ or directly from the matrix accompanied by a reduction in hardness. It has a BCT (body-centered-tetragonal) structure, and is incoherent with the matrix. As these particles are no longer coherent with the matrix, hardness is lower than at the stage when coherent was present. Over-aging continues with the growth of these particles controlled by diffusion. Variation of hardness with aging time is shown in figure-11.
The general sequence of precipitation in binary Al-Cu alloys can represented as:
Supersaturated α → GP1 zones → GP2 zones (θ” phase) → θ’ phase → θ phase (CuAl2)
Most precipitation-hardening systems operate in a similar way, peak hardness usually being attained in the later stages of coherency or at the onset of incoherency. It is quite common for a coherent precipitate to form and then lose coherency when the particle grows to a critical size. However, in some systems there is no evidence of coherency strains, and the fine particles appear to act alone as impediments to dislocation movements, for example – systems with dispersion strengthening.