Blast Furnace
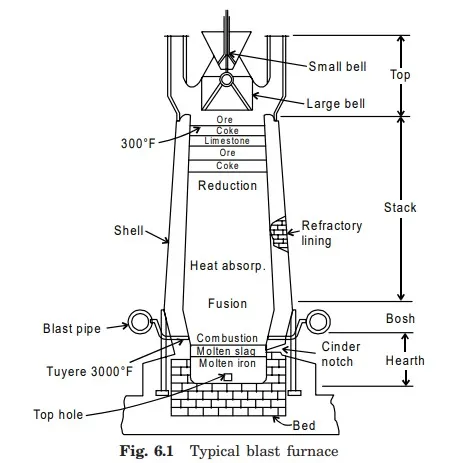
After mining, various kinds of iron ores are brought to the blast furnace which is the starting process for refining iron ores or mined ores and for the poduction of pig iron. Blast furnace was invented in 14th century. A typical blast furnace along with its various parts is shown in Fig. 6.1. It is large steel shell about 9 mt. in diameter which is lined with heat resistant bricks. It is set on the top of brick foundation. There are four major parts of blast furnace from bottom to top hearth, bosh, stack and top. The hearth acts as a storage region for molten metal and molten slag. The charge of blast furnace possesses successive layers of iron ore, scrap, coke, and limestone and some steel scrap which is fed from the top of the furnace. Iron ore exists as an aggregate of iron-bearing minerals. These mineral aggregates are oxides of iron called hematite, limonite, and magnetite. They all contribute to the smelting process. Hematite is a red ore and contains about 70% iron. Limonite is a hydrated oxide and contains about 60% iron. Magnetite is a magnetic oxide and contains about 72% iron. It takes about 1.6 tons of iron ore, 0.65 ton of coke, 0.2 ton of lime-stone and about 0.05 ton of scrap iron and steel to produce 1 ton of pig iron. For burning this charge, about 4 tons of air is required. The impurities or other minerals are present in the ore. These impurities may be silicon, sulfur, phosphorus, manganese, calcium, titanium, aluminum, and magnesium. The amounts of silicon, phosphorus, and sulfur present will determine the purification process used in the manufacture of the steel. The output from the furnace in form of pig iron is collected in large ladles from the tap hole existing at lower portion of furnace. As the coke burns, aided by the air forced into the furnace, the ore melts and collects in the hearth. As the melting process proceeds, the entire mass settles and thus makes room for the addition of charges at the top. While the melting is going on, the limestone forms a slag with the impurities. The second component which makes up the charge is the coke which is made from coal. Coke must be dust proof, not too combustible, and strong, since it must support the charge. It supplies the heat which reduces the ore and melts the iron. The iron picks up carbon from the coke and impurities from the ore. The amount of carbon picked up by the iron is more than is needed in the production of steel. The carbon becomes part of the pig iron used in the making of steel. The control of this carbon during the subsequent processes determines the properties of the steel. The manufacture of coke from bituminous coal is a distillation process. The impurities are driven off leaving coke.

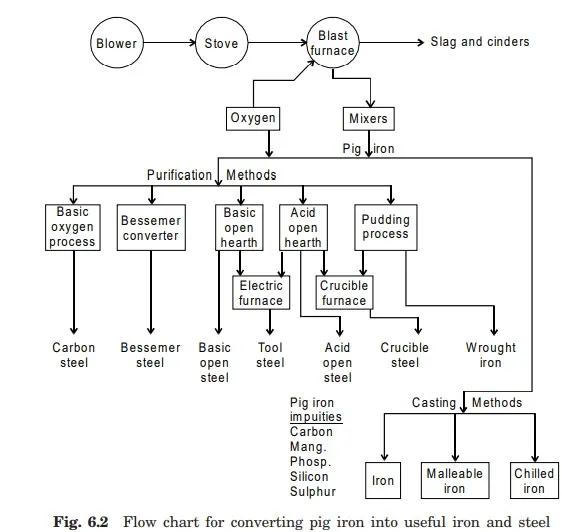
The pig iron is then processed for purification work for production of various kinds of iron and steel in form of ingots (large sections) using different furnaces. Fig 6.2 shows a flow chart for production of different kinds of iron and steel. The steel ingots can be further processed in rolling mill or blooming mill to produce different structural shapes and sections of steel. Fig 6.3 represents the entire process for production of market form of steel supply of different shapes or sections.