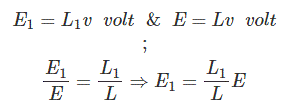
Applications of Potentiometer
There are many different uses of a potentiometer. The three main applications of a potentiometer are:
Comparing EMF of Battery Cells
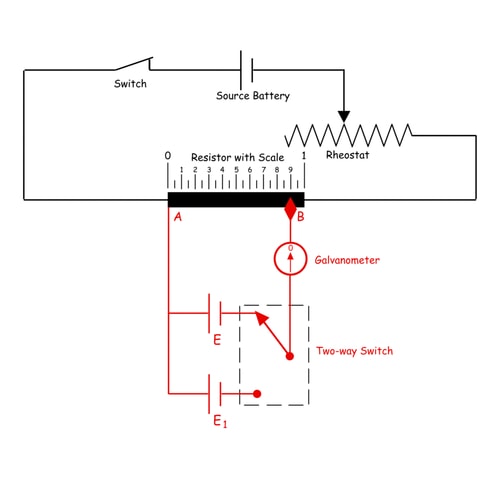
One of the main uses of a potentiometer is to compare the emf of one battery cell with a standard battery cell. Let’s take a cell whose emf is to be compared with a standard cell. The positive terminal of the cell and the same of the standard cell are joined together with the fixed end of the potentiometer resistor. The negative terminal of both cells is joined with the galvanometer in turn through a two-way switch. The other end of the galvanometer is connected to a sliding contact on the resistor. Now by adjusting sliding contact on the resistor, it is found that the null deflection of galvanometer comes for the first cell at a length of L on the scale. After positioning the two-way switch to the second cell and then by adjusting the sliding contact, it is found that the null deflection of galvanometer comes for that cell at a length of L1 on the scale. The first cell is a standard cell and its emf is E. The second cell is an unknown cell whose emf is E1. Now as per above explanation, we can write
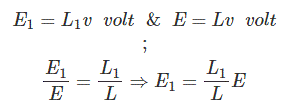
As the emf of the standard cell is known, hence emf of the unknown cell can easily be determined.
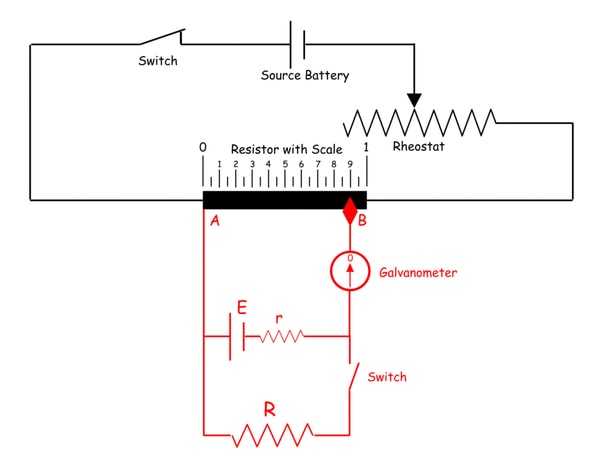
Measuring Internal Resistance of A Battery Cell
In this process, one battery is connected across the resistor of a potentiometer through a galvanometer as shown in the figure below. One resistance of known value (R) is connected across the battery through a switch. First, we keep the switch open and adjust the sliding contact of the potentiometer resistor to make the galvanometer current zero. Once the galvanometer shows zero deflection from its null point we take the position of the sliding contact tip on the resistor scale. Say this is L1.
Now we make the switch on. At that condition, a circulating current starts flowing through the battery cell as well as the resistance (R). As a result, there is a voltage drop in the battery itself due to its internal resistance. So now the voltage across the battery cell would be a little bit less than its open circuit voltage or emf of the cell. Now again we adjust the sliding contact on the transistor to make the galvanometer current zero and once it becomes zero that is zero deflection is indicated in the galvanometer, we take the position of the sliding contact tip on the resistor scale and say it is L2.
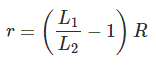
The internal resistance of the battery cell can be found out by using this below shown formula.
Where r is the internal resistance of the battery cell.