Measurement Standards
A Historical Perspective Many early standards were based on the human body: the length of man’s hand, the width of his thumb, the distance between outstretched fingertips, the length of one’s foot, a certain number of paces, etc. In the beginning, while groups were small, such standards were convenient and uniform enough to serve as the basis for measurements.
The logical person to impose a single standard was the ruler of the country — hence, our own 12-inch or other short measuring stick is still called a ruler. The establishment of measurement standards thus became the prerogative of the king or emperor, and this right has since been assumed by all governments.
History is replete with examples that show the importance of measurements and standards. In a report to the U.S. Congress in 1821, John Quincy Adams said, “Weights and measures may be ranked among the necessaries to every individual of human society.” Our founding fathers thought them so important that the United States Constitution expressly gives the Congress the power to fix uniform standards of weights and measures. The need for weights and measures (standards) dates back to earliest recorded history and are even mentioned in the Old Testament of the Bible. Originally, they were locally decreed to serve the parochial needs of commerce, trade, land division, and taxation. Because the standards were defined by local or regional authorities, differences arose that often-caused problems in commerce and early scientific investigation. The rapid growth of science in the late 17th century highlighted a number of serious deficiencies in the system of units then in use and, in 1790, led the French National Assembly to direct the French Academy of Sciences to “deduce an invariable standard for all measures and all the weights.” The Academy proposed a system of units, the metric system, to define the unit of length in terms of the earth’s circumference, with the units of volume and mass being derived from the unit of length. Additionally, they proposed that all multiples of each unit be a multiple of 10.
In 1875, the U.S. and 16 other countries signed the “Treaty of the Meter,” establishing a common set of units of measure. It also established an International Bureau of Weights and Measures (called the BIPM). That bureau is located in the Parisian suburb of Sèvres. It serves as the worldwide repository of all the units that maintain our complex international system of weights and measures. It is through this system that compatibility between measurements made thousands of miles apart is currently maintained.
The system of units set up by the BIPM is based on the meter and kilogram instead of the yard and the pound. It is called the Système International d’Unités (SI) or the International System of Units. It is used in almost all scientific work in the U.S. and is the only system of measurement units in most countries of the world today.
Even a common system of units does not guarantee measurement agreement, however. Therein lies the crux of the problem. We must make measurements, and we must know how accurately (or, to be more correct, with what uncertainty) we made those measurements. In order to know that, there must be standards. Even more important, everyone must agree on the values of those standards and use the same standards.
As the level of scientific sophistication improved, the basis for the measurement system changed dramatically. The earliest standards were based on the human body, and then attempts were made to base them on “natural” phenomena. At one time, the basis for length was supposed to be a fraction of the circumference of the earth but it was “maintained” by the use of a platinum/iridium bar. Time was maintained by a pendulum clock but was defined as a fraction of the day and so on. Today, the meter is no longer defined by an artifact. Now, the meter is the distance that light travels in an exactly defined fraction of a second. Since the speed of light in a vacuum is now defined as a constant of nature with a specified numerical value (299, 792, 458 m/s), the definition of the unit of length is no longer independent of the definition of the unit of time.
Prior to 1960, the second was defined as 1/86,400th of a mean solar day. Between 1960 and 1967, the second was defined in terms of the unit of time implicit in the calculation of the ephemerides: “The second is the fraction 1/31, 556, 925.9747 of the tropical year for January 0 at 12 hours of ephemeris time.” With the advent of crystal oscillators and, later, atomic clocks, better ways were found of defining the second. This, in turn, allowed a better understanding of things about natural phenomena that would not have been possible before. For example, it is now known that the earth does not rotate on its axis in a uniform manner. In fact, it is erratically slowing down. Since the second is maintained by atomic clocks it is necessary to add “leap seconds” periodically so that the solar day does not gradually change with respect to the time used every day. It was decided that a constant frequency standard was preferred over a constant length of the day.
What Are Standards?
One problem with standards is that there are several kinds. In addition to “measurement standards,” there are “standards of practice or protocol standards” that are produced by the various standards bodies such as the International Organization for Standardization (ISO), the International Electrotechnical Commission (IEC), the American National Standards Institute (ANSI), and the Standards Council of Canada (SCC). See Figure 5.1.
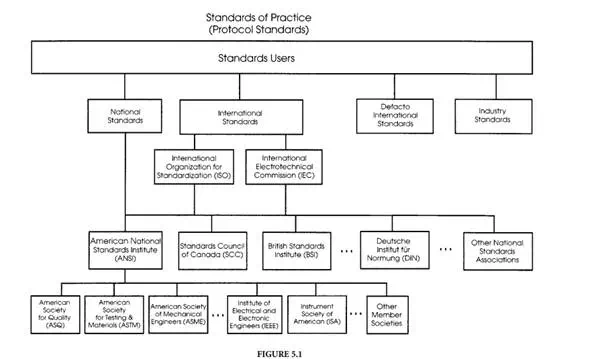
Standards of Practice (Protocol Standards)
These standards define everything from the dimensions and electrical characteristics of a flashlight battery to the shape of the threads on a machine screw and from the size and shape of an IBM punched card to the Quality Assurance Requirements for Measuring Equipment. Such standards can be defined as documents describing the operations and processes that must be performed in order for a particular end to be achieved. They are called a “protocol” by Europeans to avoid confusion with a physical standard.
Legal Metrology
The application of measurement standards to the control of the daily transactions of trade and commerce is known as Legal Metrology; within the U.S., it is more commonly known as Weights and Measures. Internationally, coordination among nations on Legal Metrology matters is, by international agreement, handled by a quasi-official body — the International Organization for Legal Metrology (OIML).
Within the U.S., domestic uniformity in legal metrology matters is the responsibility of National Institute of Standards and Technology (NIST) acting through its Office of Weights and Measures. Actual enforcement is the responsibility of each of the 50 states and the various territories. These, in turn, generally delegate the enforcement powers downward to their counties and, in some cases, to large cities.
Forensic Metrology
Forensic Metrology is the application of measurements and hence measurement standards to the solution and prevention of crime. It is practiced within the laboratories of law enforcement agencies throughout the world. Worldwide activities in Forensic Metrology are coordinated by Interpol (International Police; the international agency that coordinates the police activities of the member nations). Within the U.S., the Federal Bureau of Investigation (FBI), an agency of the Department of Justice, is the focal point for most U.S. forensic metrology activities.
Standard Reference Materials
Another type of standard that should be mentioned here are Standard Reference Materials (SRM). Standard Reference Materials are discrete quantities of substances or minor artifacts that have been certified as to their composition, purity, concentration, or some other characteristic useful in the calibration of the measurement devices and the measurement processes normally used in the process control of those substances. SRMs are the essential calibration standards in stoichiometry (the metrology of chemistry).
In the U.S., the National Institute of Standards and Technology (NIST), through its Standard Reference Materials Program, offers for sale over 1300 SRMs. These range from ores to pure metals and alloys. They also include many types of gases and gas mixtures; and many biochemical substances and organic compounds. Among the artifact devices available are optical filters with precise characteristics and standard lamps with known emission characteristics.