Multiplication Factors
Since it is inconvenient to use whole units in many cases, a set of multiplication factors has been defined that can be used in conjunction with the units to bring a value being measured to a more reasonable size. It would be difficult to have to refer to large distances in terms of the meter; thus, one defines longer distances in terms of kilometres. Short distances are stated in terms of millimetres, micrometres, nanometres, etc. See Appendix 1, Table 4.
Defining Terms
Most of the definitions in this listing were taken from the International Vocabulary of Basic and General Terms in Metrology, published by the ISO, 1993 (VIM) [7]. They are indicated by the inclusion (in brackets) of their number designation in the VIM. The remainder of the definitions are not intended to
represent any official agency but are ones widely accepted and are included to help in the understanding of this material. More detailed and rigorous definitions can be found in other works available from ANSI, IEC, ISO, and NIST. Words enclosed in parentheses “(…)” may be omitted from the term if it is unlikely that such omission will cause confusion.
Accuracy of measurement [3.5]:*
The closeness of the agreement between the result of a measurement and a true value of the measurand.
NOTES:
1. Accuracy is a qualitative concept.
2. The term precision should not be used for accuracy. (Precision only implies repeatability.)
Note, that to say an instrument is accurate to 5% (a common way of stating it) is wrong. One would not find such an instrument very useful if it, in fact, were only accurate 5% of the time. What is meant when such a statement is made is that the instrument’s inaccuracy is less than 5% and it is accurate to better than 95%. Unfortunately, this statement is almost as imprecise as “accurate to 5%.” An instrument would not be useful if it were accurate only 95% of the time; but this is not what is implied by “5% accuracy.” What is meant is that, (almost) all of the time, its indication is within 5% of the “true” value.
Calibration [6.11]:
A set of operations that establish, under specified conditions, the relationship between values of quantities indicated by a measuring instrument or measuring system, or values represented by a material measure or a reference material, and the corresponding values realized by standards.
NOTES:
1. The result of a calibration permits either the assignment of values of measurands to the indicators or the determination of corrections with respect to indications.
2. A calibration can also determine other metrological properties, such as the effect of influence quantities.
3. The result of a calibration can be recorded in a document, sometimes called a calibration certificate or a calibration report.
Calibration Laboratory:
A work space, provided with test equipment, controlled environment and trained personnel, established for the purpose of maintaining proper operation and accuracy of measuring and test equipment. Calibration laboratories typically perform many routine calibrations, often on a production-line basis.
Certified Reference Material (CRM) [6.14]:
A reference material, accompanied by a certificate, one or more of whose property values are certified by a procedure that established traceability to an accurate realization of the unit in which the property values are expressed, and for which each certified value is accompanied by an uncertainty at a stated level of confidence.
1. The definition of a reference material is given elsewhere in this vocabulary.
2. CRMs are generally prepared in batches for which the property values are determined within stated uncertainty limits by measurements on samples representative of the entire batch.
3. The certified properties of certified reference materials are sometimes conveniently and reliably realized when the material is incorporated into a specifically fabricated device, e.g., a substance of known triple-point into a triple-point cell, a glass of known optical density into a transmission filter, spheres of uniform particle size mounted on a microscope slide. Such devices can also be considered CRMs.
4. All CRMs lie within the definition of “measurement standards” given in the International Vocabulary of basic and general terms in metrology (VIM).
5. Some RMs and CRMs have properties that, because they cannot be correlated with an established chemical structure or for other reasons, cannot be determined by exactly defined physical and chemical measurement methods. Such materials include certain biological materials such as vaccines to which an International unit has been assigned by the World Health Organization. This definition, including the Notes, is taken from ISO Guide 30:1992.
Coherent (derived) unit (of measurement) [1.10]:
A derived unit of measurement that may be expressed as a product of powers of base units with the proportionality factor one (1).
NOTE:
Coherency can be determined only with respect to the base units of a particular system. A unit can be coherent with respect to one system but not to another.
Coherent system of units (of measurement) [1.11]:
A system of units of measurement in which all of the derived units are coherent.
Conservation of a (measurement) standard [6.12]:
A set of operations, necessary to preserve the metrological characteristics of a measurement standard within appropriate limits.
NOTE:
The operations commonly include periodic calibration, storage under suitable conditions, and care in use.
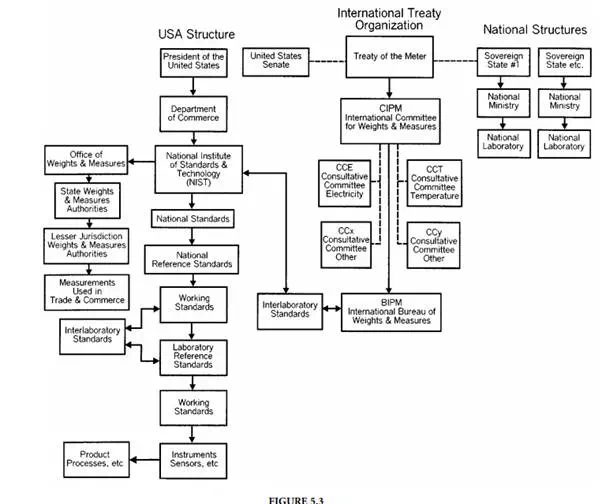
Interlaboratory Standard:
A device that travels between laboratories for the sole purpose of relating the magnitude of the physical unit represented by the standards maintained in the respective laboratories.
International (measurement) standard [6.2]:
A standard recognized by an international agreement to serve internationally as the basis for assigning values to other standards of the quantity concerned.
International System of Units (SI) [1.12]:
The coherent system of units adopted and recommended by the General Conference on Weights and Measures (CGPM).
NOTE:
The SI is based at present on the following seven base units: meter, kilogram, second, ampere, kelvin, mole, and candela.
Measurand [2.6]:
A particular quantity subject to measurement.
EXAMPLE: Vapor pressure of a given sample of water at 20°C.
NOTE: The specification of a measurand may require statements about quantities such as time, temperature, and pressure.
Measurement [2.1]:
A set of operations having the object of determining a value of a quantity.
NOTE: The operations may be performed automatically.
Method of Measurement [2.4]: A logical sequence of operations, described generically, used in the performance of measurements.
NOTE: Methods of measurement may be qualified in various ways, such as:
· Substitution method
· Differential method
· Null method
Metrology [2.2]:
The science of measurement.
NOTE: Metrology includes all aspects, both theoretical and practical, with reference to measurements, whatever their uncertainty, and in whatever fields of science or technology they occur.
National (measurement) Standard [6.3]:
A standard recognized by a national decision to serve, in a country, as the basis for assigning values to other standards of the quantity concerned.
National Reference Standard:
A standard maintained by national laboratories such as the National Institute of Standards and Technology (NIST) in Gaithersburg, MD; the National Research Council (NRC) located in Ottawa, Canada; the National Physical Laboratory (NPL) in Teddington, U.K.; the Physikalisch-Technische Bundesanstalt (PTB) at Braunschweig, Germany; and which are the legal standards of their respective countries.
National Institute of Standards and Technology (NIST):
The U.S. national standards laboratory, responsible for maintaining the physical standards upon which measurements in the U.S. are based.
Primary Standard [6.4]:
A standard that is designated or widely acknowledged as having the highest metrological qualities and whose value is accepted without reference to other standards of the same quantity.
NOTE: The concept of primary standard is equally valid for base quantities and derived quantities.
Principle of Measurement [2.3]:
The scientific base of a measurement.
EXAMPLES:
· The thermoelectric effect applied to the measurement of temperature
· The Josephson effect applied to the measurement of electric potential difference
· The Doppler effect applied to the measurement of velocity
· The Raman effect applied to the measurement of the wave number of molecular vibrations
Reference Standard [6.6]:
A standard, generally having the highest metrological quality available at a given location or in a given organization, from which measurements made there are derived.