Blue Skies and Red Sunsets
The sun emits light waves with a range of frequencies. Some
of these frequencies fall within the visible light spectrum and thus
are detectable by the human eye. Since sunlight consists of light with the
range of visible light frequencies, it appears white. This white light is incident towards Earth and illuminates both our
outdoor world and the atmosphere that surrounds our planet. As discussed earlier in
Lesson 2, the interaction of visible light with matter
will often result in the absorption of specific frequencies of light. The
frequencies of visible light that are not absorbed are either transmitted (by
transparent materials) or reflected (by opaque materials). As we sight at
various objects in our surroundings, the color that
we perceive is dependent upon the color(s) of
light that are reflected or transmitted by those objects to our eyes. So if we
consider a green leaf on a tree, the atoms of the chlorophyll molecules in the
leaf are absorbing most of the frequencies of visible light (except for green)
and reflecting the green light to our eyes. The leaf thus appears green. And as
we view the black asphalt street, the atoms of the asphalt are absorbing all
the frequencies of visible light and no light is reflected to our eyes. The
asphalt street thus appears black (the absence of color). In this manner, the interaction of sunlight with matter contributes to
the color appearance of our surrounding
world. In this part of Lesson 2, we will focus on the interaction of sunlight
with atmospheric particles to produce blue skies and red sunsets. We will
attempt to answer these two questions:
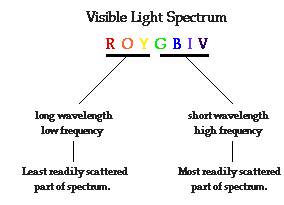
· Why are
the skies blue?
· Why are
the sunsets red?
Why are the skies blue?
The interaction of sunlight with matter can result in one of
three wave behaviors: absorption,
transmission, and reflection. The atmosphere is a gaseous
sea that contains a variety of types of particles; the two most common types of
matter present in the atmosphere are gaseous nitrogen and oxygen. These
particles are most effective in scattering the higher frequency and shorter
wavelength portions of the visible light spectrum. This scattering process
involves the absorption of a light wave by an atom followed by reemission of a
light wave in a variety of directions. The amount of multidirectional
scattering that occurs is dependent upon the frequency of the light. (In fact,
it varies according to f4.) Atmospheric nitrogen and oxygen scatter
violet light most easily, followed by blue light, green light, etc. So as white
light (ROYGBIV) from the sun passes through our atmosphere, the high
frequencies (BIV) become scattered by atmospheric particles while the lower
frequencies (ROY) are most likely to pass through the atmosphere without a
significant alteration in their direction. This scattering of the higher
frequencies of light illuminates the skies with light on the BIV end of the
visible spectrum. Compared to blue light, violet light is most easily scattered
by atmospheric particles. However, our eyes are more sensitive to light with
blue frequencies. Thus, we view the skies as being blue in color.
Why
are sunsets red?
Meanwhile, the light that is not scattered is able to pass
through our atmosphere and reach our eyes in a rather non-interrupted path. The
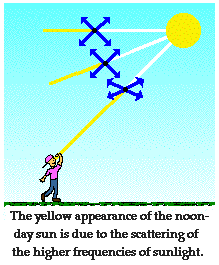
lower frequencies of sunlight (ROY) tend to reach our eyes as we sight directly
at the sun during midday. While sunlight consists of the entire range of
frequencies of visible light, not all frequencies are equally intense. In fact,
sunlight tends to be most rich with yellow light frequencies. For these
reasons, the sun appears yellow during midday due to the direct passage of
dominant amounts of yellow frequencies through our atmosphere and to our eyes.
The appearance of the sun changes with the time of day. While
it may be yellow during midday, it is often found to gradually turn color as it approaches sunset. This can be explained
by light scattering. As the sun approaches the horizon line, sunlight must
traverse a greater distance through our atmosphere; this is demonstrated in the
diagram below.
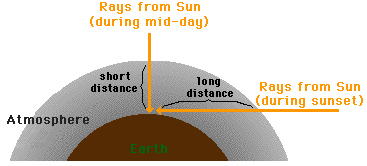
As the path that sunlight takes through our atmosphere
increases in length, ROYGBIV encounters more and more atmospheric particles.
This results in the scattering of greater and greater amounts of yellow light.
During sunset hours, the light passing through our atmosphere to our eyes tends
to be most concentrated with red and orange frequencies of light. For this
reason, the sunsets have a reddish-orange hue. The effect of a red sunset
becomes more pronounced if the atmosphere contains more and more particles. The
presence of sulfur aerosols (emitted as an
industrial pollutant and by volcanic activity) in our atmosphere contributes to
some magnificent sunsets (and some very serious environmental problems).
The Wonders of Physics

Photograph of Maui sunset by Becky Henderson