Measuring the Quantity of Heat
On the previous page, we
learned what heat does to an object when it is gained or released. Heat gains
or losses result in changes in temperature, changes in state or the performance
of work. Heat is a transfer of energy. When gained or lost by an object, there
will be corresponding energy changes within that object. A change in
temperature is associated with changes in the average kinetic energy of the
particles within the object. A change in state is associated with changes in
the internal potential energy possessed by the object. And when work is done,
there is an overall transfer of energy to the object upon which the work is
done. In this part of Lesson 2, we will investigate the question How does one measure the quantity of heat
gained or released by an object?
Specific Heat Capacity
Suppose that several objects composed of different materials
are heated in the same manner. Will the objects warm up at equal rates? The
answer: most likely not. Different materials would warm up at different rates
because each material has its own specific heat capacity. The specific heat capacity refers to
the amount of heat required to cause a unit of mass (say a gram or a kilogram)
to change its temperature by 1°C. Specific heat capacities of various materials
are often listed in textbooks. Standard metric units are Joules/kilogram/Kelvin
(J/kg/K). More commonly used units are J/g/°C. Use the widget below to
view specific heat capacities of various materials. Simply type in the name of
a substance (aluminum, iron, copper, water, methanol, wood, etc.) and click on
the Submit button; results will be displayed in a separate window.
The specific heat capacity of solid aluminum (0.904 J/g/°C) is different than
the specific heat capacity of solid iron (0.449 J/g/°C). This means that it
would require more heat to increase the temperature of a given mass of aluminum
by 1°C compared to the amount of heat required to increase the temperature of
the same mass of iron by 1°C. In fact, it would take about twice as much heat
to increase the temperature of a sample of aluminum a given amount compared to
the same temperature change of the same amount of iron. This is because the
specific heat capacity of aluminum is nearly twice the value of iron.
Heat capacities are listed on a per gram or per
kilogram basis. Occasionally, the value is listed on a per mole  basis, in which case it is called the molar heat capacity. The fact that
they are listed on a per amount basis is
an indication that the quantity of heat required to raise the temperature of a
substance depends on how much substance there is. Any person who has boiled a
pot of water on a stove, undoubtedly know this truth. Water boils at 100°C at
sea level and at slightly lowered temperatures at higher elevations. To bring a
pot of water to a boil, its temperature must first be raised to 100°C. This
temperature change is achieved by the absorption of heat from the stove burner.
One quickly notices that it takes considerably more time to bring a full pot of
water to a boil than to bring a half-full of water to a boil. This is because
the full pot of water must absorb more heat to result in the same temperature
change. In fact, it requires twice as much heat to cause the same temperature
change in twice the mass of water.
basis, in which case it is called the molar heat capacity. The fact that
they are listed on a per amount basis is
an indication that the quantity of heat required to raise the temperature of a
substance depends on how much substance there is. Any person who has boiled a
pot of water on a stove, undoubtedly know this truth. Water boils at 100°C at
sea level and at slightly lowered temperatures at higher elevations. To bring a
pot of water to a boil, its temperature must first be raised to 100°C. This
temperature change is achieved by the absorption of heat from the stove burner.
One quickly notices that it takes considerably more time to bring a full pot of
water to a boil than to bring a half-full of water to a boil. This is because
the full pot of water must absorb more heat to result in the same temperature
change. In fact, it requires twice as much heat to cause the same temperature
change in twice the mass of water.
Specific heat capacities are also listed on a per K or a per °C basis. The
fact that the specific heat capacity is listed on a per degree basis is
an indication that the quantity of heat required to raise a given mass of
substance to a specific temperature depends upon the change in temperature
required to reach that final temperature. In other words, it is not the final
temperature that is of importance, it is the overall temperature change. It
takes more heat to change the temperature of water from 20°C to 100°C (a change
of 80°C) than to increase the temperature of the same amount of water from 60°C
to 100°C (a change of 40°C). In fact, it requires twice as much heat to change
the temperature of a given mass of water by 80°C compared to the change of
40°C. A person who wishes to bring water to a boil on a stovetop more quickly
should begin with warm tap water instead of cold tap water.
This discussion of specific heat capacity deserves one final
comment. The term specific heat capacity is somewhat of a misnomer. The term implies that substances may have the ability to contain a thing called
heat. As has been previously discussed, heat is not something that is
contained in an object. Heat is something that is transferred to or from an
object. Objects contain energy in a variety of forms. When that energy is
transferred to other objects of different temperatures, we refer to transferred
energy as heat orthermal energy. While it's not likely to catch on, a more
appropriate term would be specific energy capacity.
Relating the Quantity of Heat to the Temperature
Change
Specific heat capacities provide a means of mathematically
relating the amount of thermal energy gained (or lost) by a sample of any
substance to the sample's mass and its resulting temperature change. The
relationship between these four quantities is often expressed by the following
equation.
Q = m•C•ΔT
 where Q is the quantity of heat transferred to or from
the object, m is the
mass of the object, C is the
specific heat capacity of the material the object is composed of, and ΔT is the resulting temperature change of the
object. As in all situations in science, a delta (∆)
value for any quantity is calculated by subtracting the initial value of the
quantity from the final value of the quantity. In this case, ΔT is equal
to Tfinal - Tinitial. When using the above
equation, the Q value can turn out to be either positive or negative. As
always, a positive and a negative result from a calculation has physical
significance. A positive Q value indicates that the object gained thermal energy
from its surroundings; this would correspond to an increase in temperature and
a positive ΔT value. A negative Q value indicates that the object released
thermal energy to its surroundings; this would correspond to a decrease in
temperature and a negative ΔT value.
where Q is the quantity of heat transferred to or from
the object, m is the
mass of the object, C is the
specific heat capacity of the material the object is composed of, and ΔT is the resulting temperature change of the
object. As in all situations in science, a delta (∆)
value for any quantity is calculated by subtracting the initial value of the
quantity from the final value of the quantity. In this case, ΔT is equal
to Tfinal - Tinitial. When using the above
equation, the Q value can turn out to be either positive or negative. As
always, a positive and a negative result from a calculation has physical
significance. A positive Q value indicates that the object gained thermal energy
from its surroundings; this would correspond to an increase in temperature and
a positive ΔT value. A negative Q value indicates that the object released
thermal energy to its surroundings; this would correspond to a decrease in
temperature and a negative ΔT value.
Knowing any three of these four quantities allows an
individual to calculate the fourth quantity. A common task in many physics
classes involves solving problems associated with the relationships between
these four quantities. As examples, consider the two problems below. The
solution to each problem is worked out for you. Additional practice can be
found in the Check Your Understanding section at the
bottom of the page.
|
Example Problem 1 |
Like any problem in physics, the solution begins by
identifying known quantities and relating them to the symbols used in the
relevant equation. In this problem, we know the following:
m = 450 g
C = 4.18 J/g/°C
Tinitial = 15°C
Tfinal = 85°C
We wish to determine the value of Q - the quantity of heat. To do so, we would use
the equation Q = m•C•ΔT. The m and the C are known; the ΔT can be determined from the initial and final
temperature.
T = Tfinal - Tinitial = 85°C -
15°C = 70.°C
With three of the four quantities of the relevant equation
known, we can substitute and solve for Q.
Q = m•C•ΔT = (450 g)•(4.18
J/g/°C)•(70.°C)
Q = 131670 J
Q = 1.3x105 J = 130 kJ (rounded to two significant digits)
|
Example Problem 2 |
Compared to the previous problem, this is a much more difficult problem. In
fact, this problem is like two problems in one. At the center of the
problem-solving strategy is the recognition that the quantity of heat lost by
the water (Qwater) equals the quantity of heat gained by the metal (Qmetal). Since the m, C and ΔTvalues of the water are known, the Qwater can be calculated. This Qwater value equals the Qmetal value. Once the Qmetal value is known, it can be used with the m and ΔT value of
the metal to calculate the Qmetal.Use of this strategy leads to the following solution:
Part 1: Determine the Heat Lost by the Water
Given:
m = 50.0 g
C = 4.18 J/g/°C
Tinitial = 88.6°C
Tfinal = 87.1°C
ΔT = -1.5°C (Tfinal - Tinitial)
Solve for Qwater:
Qwater = m•C•ΔT = (50.0 g)•(4.18
J/g/°C)•(-1.5°C)
Qwater = -313.5 J (unrounded)
(The - sign indicates that heat is lost by the water)
Part 2: Determine the value of Cmetal
Given:
Qmetal = 313.5 J (use a + sign since the metal is
gaining heat)
m = 12.9 g
Tinitial = 26.5°C
Tfinal = 87.1°C
ΔT = (Tfinal - Tinitial )
Solve for Cmetal:
Rearrange Qmetal = mmetal•Cmetal•ΔTmetal to obtain
Cmetal = Qmetal / (mmetal•ΔTmetal)
Cmetal = Qmetal / (mmetal•ΔTmetal)
= (313.5 J)/[(12.9 g)•(60.6°C)]
Cmetal = 0.40103 J/g/°C
Cmetal = 0.40 J/g/°C (rounded to two significant digits)
Heat and Changes of State
The discussion above and the accompanying equation (Q =
m•C•∆T) relates the heat gained or lost by an object to the resulting
temperature changes of that object. As we have learned,
sometimes heat is gained or lost but there is no temperature change. This is
the case when the substance is undergoing a state change. So now we must
investigate the mathematics related to changes in state and the quantity of
heat.
To begin the discussion, let's consider the various state
changes that could be observed for a sample of matter. The table below lists
several state changes and identifies the name commonly associated with each
process.
|
Process |
Change of State |
|
Melting |
Solid to Liquid |
|
Freezing |
Liquid to Solid |
|
Vaporization |
Liquid to Gas |
|
Condensation |
Gas to Liquid |
|
Sublimation |
Solid to Gas |
|
Deposition |
Gas to Solid |
In the case of melting, boiling and sublimation, energy would have to be added
to the sample of matter in order to cause the change of state. Such state
changes are referred to as being endothermic. Freezing, condensation and deposition are exothermic; energy is released by the
sample of matter when these state changes occur. So one might notice that a
sample of ice (solid water) undergoes melting when it is placed on or near a
burner. Heat is transferred from the burner to the sample of ice; energy is
gained by the ice causing the change of state. But how much energy would be
required to cause such a change of state? Is there a mathematical formula that
might help in determining the answer to this question? There most certainly is.
The amount of energy required to change the state of a sample
of matter depends on three things. It depends upon what the substance is, on
how much substance is undergoing the state change, and upon what state change
that is occurring. For instance, it requires a different amount of energy to
melt ice (solid water) compared to melting iron. And it requires a different
amount of energy to melt ice (solid water) as it does to vaporize the same
amount of liquid water. And finally, it requires a different amount of energy
to melt 10.0 grams of ice compared to melting 100.0 grams of ice. The
substance, the process and the amount of substance are the three variables that
affect the amount of energy required to cause a specific change in state. Use
the widget below to investigate the effect of the substance and the process
upon the energy change. (Note that the Heat of Fusion is the energy change
associated with the solid-liquid state change.)
The values for the specific heat of fusion and the specific heat of
vaporization are reported on a per amount basis. For
instance, the specific heat of fusion of water is 333 J/gram. It takes 333 J of
energy to melt 1.0 gram of ice. It takes 10 times as much energy - 3330 J - to
melt 10.0 grams of ice. Reasoning in this manner leads to the following
formulae relating the quantity of heat to the mass of the substance and the
heat of fusion and vaporization.
For melting and freezing: Q = m•ΔHfusion
For vaporization and condensation: Q = m•ΔHvaporization
where Q represents
the quantity of energy gained or released during the process, m represents the mass of the sample, ΔHfusion represents
the specific heat of fusion (on a per gram basis) and ΔHvaporizationrepresents
the specific heat of vaporization (on a per gram basis). Similar to the
discussion regarding Q = m•C•ΔT, the values of Q can be either positive or negative. Values of Q are
positive for the melting and vaporization process; this is consistent with the
fact that the sample of matter must gain energy in order to melt or vaporize.
Values of Q are negative for the freezing and condensation process; this is
consistent with the fact that the sample of matter must lose energy in order to
freeze or condense.
As an illustration of how these equations can be used,
consider the following two example problems.
|
Example Problem 3 |
The equation relating the mass (48.2 grams), the heat of
fusion (333 J/g), and the quantity of energy (Q) isQ = m•ΔHfusion.
Substitution of known values into the equation leads to the answer.
Q = m•ΔHfusion = (48.2 g)•(333 J/g)
Q = 16050.6 J
Q = 1.61 x 104 J = 16.1 kJ (rounded to three significant digits)
Example Problem 3 involves a rather straightforward,
plug-and-chug type calculation. Now we will try Example Problem 4, which will
require a significant deeper level of analysis.
|
Example Problem 4 |
In this problem, the ice is melting and the liquid water is
cooling down. Energy is being transferred from the liquid to the solid. To melt
the solid ice, 333 J of energy must be transferred for every gram of ice. This
transfer of energy from the liquid water to the ice will cool the liquid down.
But the liquid can only cool as low as 0°C - the freezing point of the water.
At this temperature the liquid will begin to solidify (freeze) and the ice will
not completely melt.
We know the following about the ice and the liquid water:
Given Info about Ice:
m = 50.0 g
ΔHfusion = 333 J/g
Given Info about Liquid Water:
C = 4.18 J/g/°C
Tinitial = 26.5°C
Tfinal = 0.0°C
ΔT = -26.5°C (Tfinal - Tinitial )
The energy gained by the ice is equal to the energy lost from
the water.
Qice = -Qliquid water
The - sign indicates that the one object gains energy and the
other object loses energy. We can calculate the left side of the above equation
as follows:
Qice = m•ΔHfusion = (50.0 g)•(333 J/g)
Qice = 16650 J
Now we can set the right side of the equation equal to
m•C•ΔT and begin to substitute in known values of C and ΔT in order
to solve for the mass of the liquid water. The solution is:
16650 J = -Qliquid water
16650 J = -mliquid water•Cliquid water•ΔTliquid
water
16650 J = -mliquid water•(4.18
J/g/°C)•(-26.5°C)
16650 J = -mliquid water•(-110.77 J/°C)
mliquid water = -(16650 J)/(-110.77 J/°C)
mliquid water = 150.311 g
mliquid water = 1.50x102 g (rounded to three significant digits)
Heating and Cooling Curves Revisited
On the previous page of Lesson 2, the heating curve of water was discussed. The heating curve showed how
the temperature of water increased over the course of time as a sample of water
in its solid state (i.e., ice) was heated. We learned that the addition of heat
to the sample of water could cause either changes in temperature or changes in
state. At the melting point of water, the addition of heat causes a
transformation of the water from the solid state to the liquid state. And at
the boiling point of water, the addition of heat causes a transformation of the
water from the liquid state to the gaseous state. These changes in state
occurred without any changes in temperature. However, the addition of heat to a
sample of water that is not at any phase change temperatures will result in a change
in temperature.
Now we can approach the topic of heating curves on a more
quantitative basis. The diagram below represents the heating curve of water.
There are five labeled sections on the plotted lines.
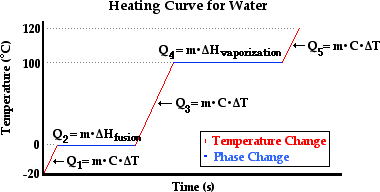
The three diagonal sections represent the changes in
temperature of the sample of water in the solid state (section 1), the liquid
state (section 3), and the gaseous state (section 5). The two horizontal
sections represent the changes in state of the water. In section 2, the sample
of water is undergoing melting; the solid is changing to a liquid. In section
4, the sample of water is undergoing boiling; the liquid is changing to a gas.
The quantity of heat transferred to the water in sections 1, 3, and 5 is
related to the mass of the sample and the temperature change by the formula Q = m•C•ΔT. And the
quantity of heat transferred to the water in sections 2 and 4 is related to the
mass of the sample and the heat of fusion and vaporization by the formulae Q = m•ΔHfusion (section
2) and Q = m•ΔHvaporization (section
4). So now we will make an effort to calculate the quantity of heat required to
change 50.0 grams of water from the solid state at -20.0°C to the gaseous state
at 120.0°C. The calculation will require five steps - one step for each section
of the above graph. While the specific heat capacity of a substance varies with
temperature, we will use the following values of specific heat in our
calculations:
Solid Water: C=2.00 J/g/°C
Liquid Water: C = 4.18 J/g/°C
Gaseous Water: C = 2.01 J/g/°C
Finally, we will use the previously reported values of
ΔHfusion (333 J/g) and ΔHvaporization (2.23
kJ/g).
Section 1: Changing
the temperature of solid water (ice) from -20.0°C to 0.0°C.
Use Q1 = m•C•ΔT
where m = 50.0 g,
C = 2.00 J/g/°C, Tinitial = -200°C, andTfinal = 0.0°C
Q1 = m•C•ΔT = (50.0 g)•(2.00
J/g/°C)•(0.0°C - -20.0°C)
Q1 = 2.00 x103 J = 2.00 kJ
Section 2: Melting
the Ice at 0.0°C.
Use Q2 = m•ΔHfusion
where m = 50.0 g
and ΔHfusion = 333 J/g
Q2 = m•ΔHfusion = (50.0 g)•(333 J/g)
Q2 = 1.665
x104 J = 16.65 kJ
Q2 = 16.7 kJ
(rounded to 3 significant digits)
Section 3: Changing
the temperature of liquid water from 0.0°C to 100.0°C.
Use Q3 = m•C•ΔT
where m = 50.0 g,
C = 4.18 J/g/°C, Tinitial = 0.0°C, and Tfinal = 100.0°C
Q3 = m•C•ΔT = (50.0 g)•(4.18
J/g/°C)•(100.0°C - 0.0°C)
Q3 = 2.09 x104 J = 20.9 kJ
Section 4: Boiling
the Water at 100.0°C.
Use Q4 = m•ΔHvaporization
where m = 50.0 g
and ΔHvaporization = 2.23 kJ/g
Q4 = m•ΔHvaporization = (50.0 g)•(2.23 kJ/g)
Q4 = 111.5 kJ
Q4 = 112 kJ
(rounded to 3 significant digits)
Section 5: Changing
the temperature of liquid water from 100.0°C to 120.0°C.
Use Q5 = m•C•ΔT
where m = 50.0 g,
C = 2.01 J/g/°C, Tinitial = 100.0°C, and Tfinal = 120.0°C
Q5 = m•C•ΔT = (50.0 g)•(2.01
J/g/°C)•(120.0°C - 100.0°C)
Q5 = 2.01 x103 J = 2.01 kJ
The total amount of heat required to change solid water (ice)
at -20°C to gaseous water at 120°C is the sum of the Q values for each section of the graph. That is,
Qtotal = Q1 + Q2 + Q3 + Q4 + Q5
Summing these five Q values and
rounding to the proper number of significant digits leads to a value of154 kJ as the
answer to the original question.
In the above example, there are several features of the solution that are worth
reflecting on:
· First: The
lengthy problem was divided into parts, with each part representing one of the
five sections of the graph. Since there were five Q values being calculated,
they were labeled as Q1, Q2, etc. This level of
organization is required in a multi-step problem such as this one.
· Second: Attention
was given to the +/- sign on ΔT. The
change in temperature (or of any quantity) is always calculated as the final
value of the quantity minus the initial value of that quantity.
· Third: Attention
was given to units throughout the course of the problem. Units of Q will either
be in Joule or kiloJoule depending on which
quantities are being multiplied. Failure to pay attention to units is a common
cause of failure in problems like these.
· Fourth: Attention
was given to significant digits throughout the course of the problem. While
this should never become the major emphasis of any problem in physics, it is
certainly a detail worth attending to.
We've learned here on this page how to calculate the quantity
of heat involved in any heating/cooling process and in any change of state
process. This understanding will be critical as we proceed to the next
page of Lesson 2 on the topic of calorimetry. Calorimetry is the
science associated with determining the changes in energy of a system by
measuring the heat exchanged with the surroundings.
Check Your Understanding
1. Water has an unusually high specific heat capacity. Which
one of the following statements logically follows from this fact?
a. Compared to other substances, hot water causes severe
burns because it is a good conductor of heat.
b. Compared to other substances, water will quickly warm up to high
temperatures when heated.
c. Compared to other substances, it takes a considerable amount of heat for a
sample of water to change its temperature by a small amount.

Answer: C
A substance with
a high specific heat capacity is a substance that requires a relative large
quantity of heat to cause a small temperature change. Because of this, water
does not change its temperature as rapidly as other substances that are heated
in the same manner; choice B does not logically follow. Specific heat capacity
should not be confused with thermal conductivity. Thermal conductivity is the
measure of the ability of a substance to conduct heat; choice A has to do with
thermal conductivity.
2. Explain why large bodies of water such as Lake Michigan
can be quite chilly in early July despite the outdoor air temperatures being
near or above 90°F (32°C).

Answer:
Lake
Michigan is a body of water with a large m value and
a large C value. It would take a lot of solar energy
absorption to increase its temperature from the cold wintry temperatures to the
higher summertime temperatures. It may take a couple of months of summer before
the heating of the large mass of water is "complete."
3. The table below describes a thermal process for a variety
of objects (indicated by red, bold-faced text). For each description, indicate
if heat is gained or lost by the object, whether the process is endothermic or
exothermic, and whether Q for the indicated object is a positive or negative
value.
|
|
Process |
Heat Gained or Heat Lost? |
Endo- or Exothermic? |
Q: + or -? |
|
a. |
An ice cube is placed into a glass of room
temperature lemonade in order to cool the beverage down. |
|
|
|
|
b. |
A
cold glass of lemonade sits on the picnic table in the hot
afternoon sun and warms up to 32°F. |
|
|
|
|
c. |
The burners on
an electric stove are turned off and gradually cool down to room temperature. |
|
|
|
|
d. |
The
teacher removes a large chunk of dry icefrom a
thermos and places it into water. The dry ice sublimes, producing gaseous
carbon dioxide. |
|
|
|
|
e. |
Water vapor in the humidified air strikes the
window and turns to a dew drop (drop of liquid water). |
|
|
|

Answer:
|
|
Process |
Heat Gained or Heat Lost? |
Endo- or Exothermic? |
Q: + or -? |
|
a. |
An ice cube is placed into a glass of room temperature lemonade in
order to cool the beverage down. |
Gained |
Endo |
+ |
|
b. |
A cold glass of lemonade sits on the picnic table in the hot afternoon sun and
warms up to 32°F. |
Gained |
Endo |
+ |
|
c. |
The burners on an electric stove are turned off and gradually cool
down to room temperature. |
Lost |
Exo |
- |
|
d. |
The teacher removes a large chunk ofdry ice from a thermos and places
it into water. The dry ice sublimes, producing gaseous carbon dioxide. |
Gained |
Endo |
+ |
|
e. |
Water vapor in the humidified air strikes the
window and turns to a dew drop (drop of liquid water). |
Lost |
Exo |
- |
4. An 11.98-gram sample of zinc metal is placed in a hot
water bath and warmed to 78.4°C. It is then removed and placed into a Styrofoam
cup containing 50.0 mL of room temperature water (T=27.0°C; density = 1.00
g/mL). The water warms to a temperature of 28.1°C. Determine the specific heat
capacity of the zinc.

Answer:0.38 J/g/°C
The water warms up and the energy it gains is equal to the
energy lost by the metal. The quantity of energy gained by the water can be
calculated as
Qwater = m•Cwater•ΔT = (50.0 g)•(4.18 J/g/°C)•(28.1°C-27.0°C) = 229.9 J
Now this 229.9 J is equal to the -Qmetal. The
specific heat capacity of the metal can be calculated by setting -229.9 J equal
to m•C•ΔT.
C = -229.9 J/(11.98 g)/(28.1°C - 78.4°C) = 0.382 J/g/°C
5. Jake grabs a can of soda from the closet and pours it over
ice in a cup. Determine the amount of heat lost by the room temperature soda as
it melts 61.9 g of ice (ΔHfusion = 333 J/g).

Answer: 20.6 kJ
Use the
equation Q = m•ΔHfusion where m=61.9 g and
ΔHfusion=333 J/g. Conversion to kiloJoule is of course optional.
6. The heat of sublimation (ΔHsublimation) of
dry ice (solid carbon dioxide) is 570 J/g. Determine the amount of heat
required to turn a 5.0-pound bag of dry ice into gaseous carbon dioxide.
(Given: 1.00 kg = 2.20 lb)

Answer: 1300 kJ (rounded from 1295 kJ)
mdry ice = 5.0 lb•(1.00 kg/2.2
lb) = 2.2727 kg
Now that the mass of dry ice is known, the Q value can be determined.
Again, attention must be given to units. Since the mass is known in kilogram,
it would be useful to express the heat of sublimation in kJ/kg. So 570 J/g is
equivalent to 570 kJ/kg. And so the answer is calculated as
Q = mdry ice • ΔHsublimation-dry ice
Q = (2.2727 kg)•(570 kJ/kg) = 1295 kJ
Q = ~1300 kg
(rounded to two significant digits)
7. Determine the amount of heat required to increase the
temperature of a 3.82-gram sample of solid para-dichlorobenzene from 24°C to
its liquid state at 75°C. Para-dichlorobenzene has a melting point of 54°C, a
heat of fusion of 124 J/g and specific heat capacities of 1.01 J/g/°C (solid
state) and 1.19 J/g/°C (liquid state).

Answer: 680 J (rounded from 684.9 J)
This problem requires three steps - calculating the Q1 for
raising the temperature of para-dichlorobenzene (abbreviated as PDCB for the
remainder of the problem) to 54°C (the melting point), calculating the Q2 for
melting the PDCB, and calculating the Q3 for raising the
temperature of the liquid PDCB to 75°C.
Q1 =(3.82 g)•(1.01
J/g/°C)•(54°C-24°C) = 115.7 J
Q2 =(3.82 g)•(124 J/g)
= 473.7 J
Q3 =(3.82 g)•(1.19
J/g/°C)•(75°C-54°C) = 95.5 J
Qtotal =
Q1 + Q2 + Q3 = 684.9 J