Methods of Heat Transfer
If you have been following along since the beginning of this
lesson, then you have been developing a progressively sophisticated
understanding of temperature and heat. You should be developing a model of
matter as consisting of particles which vibrate (wiggle about a fixed
position), translate (move from one location to another) and even rotate
(revolve about an imaginary axis). These motions give the particles kinetic
energy. Temperature is a measure of the average amount of kinetic energy
possessed by the particles in a sample of matter. The more the particles
vibrate, translate and rotate, the greater the temperature of the object. You
have hopefully adopted an understanding of heat as a flow of energy from a
higher temperature object to a lower temperature object. It is the temperature
difference between the twoneighboring objects that causes this heat transfer. The heat transfer
continues until the two objects have reached thermal equilibrium and are at the
same temperature. The discussion of heat transfer has been structured around
some everyday examples such as the cooling of a hot mug of coffee and the
warming of a cold can of pop. Finally, we have explored a thought experiment in
which a metal can containing hot water is placed within a Styrofoam cup containing
cold water. Heat is transferred from the hot water to the cold water until both
samples have the same temperature.
 Now we should probe some of the following questions:
Now we should probe some of the following questions:
· What is
happening at the particle level when energy is being transferred between two
objects?
· Why is thermal equilibrium always established when two
objects transfer heat?
· How does
heat transfer work within the bulk of an object?
· Is there
more than one method of heat transfer? If so, then how are they similar and
different than one another?
Conduction - A Particle View
Let's begin our discussion by returning to our thought
experiment in which a metal can containing hot water was placed within a
Styrofoam cup containing cold water. Heat is transferred from the hot water to
the cold water until both samples have the same temperature. In this instance,
the transfer of heat from the hot water through the metal can to the cold water
is sometimes referred to as conduction.
Conductive heat flow involves the transfer of heat from one location to another
in the absence of any material flow. There is nothing physical or material
moving from the hot water to the cold water. Only energy is transferred from
the hot water to the cold water. Other than the loss of energy, there is
nothing else escaping from the hot water. And other than the gain of energy,
there is nothing else entering the cold water. How does this happen? What is
the mechanism that makes conductive heat flow possible?
 A question like this is a particle-level question. To understand the
answer, we have to think about matter as consisting of tiny particles atoms,
molecules and ions. These particles are in constant motion; this gives them
kinetic energy. As mentioned previously in this lesson, these particles
move throughout the space of a container, colliding with each other and with
the walls of their container. This is known as translational kinetic energy and
is the main form of kinetic energy for gases and liquids. But these particles
can also vibrate about a fixed position. This gives the particles vibrational
kinetic energy and is the main form of kinetic energy for solids. To put it
more simply, matter consists of little wigglers and little bangers. The
wigglers are those particles vibrating about a fixed position. They possess
vibrational kinetic energy. The bangers are those particles that move through
the container with translational kinetic energy and collide with the container
walls.
A question like this is a particle-level question. To understand the
answer, we have to think about matter as consisting of tiny particles atoms,
molecules and ions. These particles are in constant motion; this gives them
kinetic energy. As mentioned previously in this lesson, these particles
move throughout the space of a container, colliding with each other and with
the walls of their container. This is known as translational kinetic energy and
is the main form of kinetic energy for gases and liquids. But these particles
can also vibrate about a fixed position. This gives the particles vibrational
kinetic energy and is the main form of kinetic energy for solids. To put it
more simply, matter consists of little wigglers and little bangers. The
wigglers are those particles vibrating about a fixed position. They possess
vibrational kinetic energy. The bangers are those particles that move through
the container with translational kinetic energy and collide with the container
walls.
The container walls represent the perimeters of a sample of
matter. Just as the perimeter
of your property(as
in real estate property) is the furthest extension of the property, so the
perimeter of an object is the furthest extension of the particles within a
sample of matter. At the perimeter, the little
bangers are colliding with
particles of another substance - the particles of the container or even the
surrounding air. Even the wigglers that are fixed in a position along the
perimeter are doing some banging. Being at the perimeter, their wiggling
results in collisions with the particles that are next to them; these are the
particles of the container or of the surrounding air.
 At this perimeter or boundary, the collisions of the little bangers and
wigglers are elastic collisions in which the total amount of kinetic energy of
all colliding particles is conserved. The net effect of these elastic
collisions is that there is a transfer of kinetic energy across the boundary to
the particles on the opposite side. The more energetic particles will lose a
little kinetic energy and the less energetic particles will gain a little
kinetic energy. Temperature is a measure of the average amount of kinetic
energy possessed by the particles in a sample of matter. So on average, there
are more particles in the higher temperature object with greater kinetic energy
than there are in the lower temperature object. So when we average all the
collisions together and apply the principles associated with elastic collisions
to the particles within a sample of matter, it is logical to conclude that the
higher temperature object will lose some kinetic energy and the lower
temperature object will gain some kinetic energy. The collisions of our little
bangers and wigglers will continue to transfer energy until the temperatures of
the two objects are identical. When this state of thermal equilibrium has been
reached, the average kinetic energy of both objects' particles is equal. At
thermal equilibrium, there are an equal number of collisions resulting in an
energy gain as there are collisions resulting in an energy loss. On average,
there is no net energy transfer resulting from the collisions of particles at
the perimeter.
At this perimeter or boundary, the collisions of the little bangers and
wigglers are elastic collisions in which the total amount of kinetic energy of
all colliding particles is conserved. The net effect of these elastic
collisions is that there is a transfer of kinetic energy across the boundary to
the particles on the opposite side. The more energetic particles will lose a
little kinetic energy and the less energetic particles will gain a little
kinetic energy. Temperature is a measure of the average amount of kinetic
energy possessed by the particles in a sample of matter. So on average, there
are more particles in the higher temperature object with greater kinetic energy
than there are in the lower temperature object. So when we average all the
collisions together and apply the principles associated with elastic collisions
to the particles within a sample of matter, it is logical to conclude that the
higher temperature object will lose some kinetic energy and the lower
temperature object will gain some kinetic energy. The collisions of our little
bangers and wigglers will continue to transfer energy until the temperatures of
the two objects are identical. When this state of thermal equilibrium has been
reached, the average kinetic energy of both objects' particles is equal. At
thermal equilibrium, there are an equal number of collisions resulting in an
energy gain as there are collisions resulting in an energy loss. On average,
there is no net energy transfer resulting from the collisions of particles at
the perimeter.
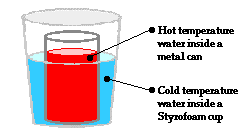 At the macroscopic level, heat is the transfer of energy from the high
temperature object to the low temperature object. At the particle level, heat
flow can be explained in terms of the net effect of the collisions of a whole bunch of little bangers.
Warming and cooling is the macroscopic result of this particle-level
phenomenon. Now let's apply this particle view to the scenario of the metal can
with the hot water positioned inside of a Styrofoam cup containing cold water.
On average, the particles with the greatest kinetic energy are the particles of
the hot water. Being a fluid, those particles move about with translational
kinetic energy and bang upon the particles of the metal can. As
the hot water particles bang upon the particles of the metal can, they transfer
energy to the metal can. This warms the metal can up. Most metals are good
thermal conductors so they warm up quite quickly throughout the bulk of the
can. The can assumes nearly the same temperature as the hot water. Being a
solid, the metal can consists of little
wigglers. The wigglers at the outer perimeter of the metal can bang upon particles in the cold water. The
collisions between the particles of the metal can and the particles of the cold
water result in the transfer of energy to the cold water. This slowly warms the
cold water up. The interaction between the particles of the hot water, the
metal can and the cold water results in a transfer of energy outward from the
hot water to the cold water. The average kinetic energy of the hot water
particles gradually decreases; the average kinetic energy of the cold-water
particles gradually increases; and eventually, thermal equilibrium would be
reached at the point that the particles of the hot water and the cold water
have the same average kinetic energy. At the macroscopic level, one would
observe a decrease in temperature of the hot water and an increase in
temperature of the cold water.
At the macroscopic level, heat is the transfer of energy from the high
temperature object to the low temperature object. At the particle level, heat
flow can be explained in terms of the net effect of the collisions of a whole bunch of little bangers.
Warming and cooling is the macroscopic result of this particle-level
phenomenon. Now let's apply this particle view to the scenario of the metal can
with the hot water positioned inside of a Styrofoam cup containing cold water.
On average, the particles with the greatest kinetic energy are the particles of
the hot water. Being a fluid, those particles move about with translational
kinetic energy and bang upon the particles of the metal can. As
the hot water particles bang upon the particles of the metal can, they transfer
energy to the metal can. This warms the metal can up. Most metals are good
thermal conductors so they warm up quite quickly throughout the bulk of the
can. The can assumes nearly the same temperature as the hot water. Being a
solid, the metal can consists of little
wigglers. The wigglers at the outer perimeter of the metal can bang upon particles in the cold water. The
collisions between the particles of the metal can and the particles of the cold
water result in the transfer of energy to the cold water. This slowly warms the
cold water up. The interaction between the particles of the hot water, the
metal can and the cold water results in a transfer of energy outward from the
hot water to the cold water. The average kinetic energy of the hot water
particles gradually decreases; the average kinetic energy of the cold-water
particles gradually increases; and eventually, thermal equilibrium would be
reached at the point that the particles of the hot water and the cold water
have the same average kinetic energy. At the macroscopic level, one would
observe a decrease in temperature of the hot water and an increase in
temperature of the cold water.
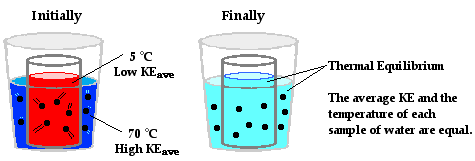
The mechanism in which heat is transferred from one object to
another object through particle collisions is known as conduction. In
conduction, there is no net transfer of physical stuff between the objects. Nothing material
moves across the boundary. The changes in temperature are wholly explained as
the result of the gains and losses of kinetic energy during collisions.
Conduction Through The Bulk of an Object
We have discussed how heat transfers from one object to
another through conduction. But how does it transfer through the bulk of an
object? For instance, suppose we pull a ceramic coffee mug out of the cupboard
and place it on the countertop. The mug is at room temperature - maybe at 26°C.
Then suppose we fill the ceramic coffee mug with hot coffee at a temperature of
80°C. The mug quickly warms up. Energy first flows into the particles at the
boundary between the hot coffee and the ceramic mug. But then it flows through
the bulk of the ceramic to all parts of the ceramic mug. How does heat conduction
occur in the ceramic itself?
The mechanism of heat transfer through the bulk of the
ceramic mug is described in a similar manner as it before. The ceramic mug
consists of a collection of orderly arranged wigglers. These are particles that
wiggle about a fixed position. As the ceramic particles at the boundary between
the hot coffee and the mug warm up, they attain a kinetic energy that is much
higher than their neighbors. As they wiggle more vigorously, they bang
into their neighbors and
increase their vibrational kinetic energy. These particles in turn begin to
wiggle more vigorously and their collisions with their neighbors increase
their vibrational kinetic energy. The process of energy transfer by means of
the little bangers continues from the particles at the
inside of the mug (in contact with the coffee particles) to the outside of the
mug (in contact with the surrounding air). Soon the entire coffee mug is warm
and your hand feels it.
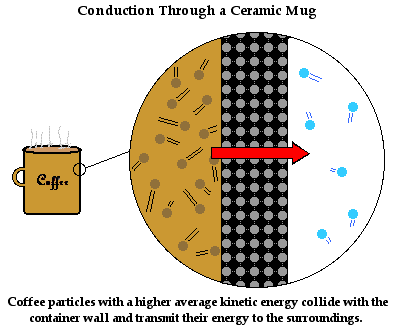
This mechanism of conduction by particle-to-particle
interaction is very common in ceramic materials such as a coffee mug. Does it
work the same in metal objects? For instance, you likely have noticed the high
temperatures attained by the metal handle of a skillet when placed upon a
stovetop. The burners on the stove transfer heat to the metal skillet. If the
handle of the skillet is metallic, it too attains a high temperature, certainly
high enough to cause a bad burn. The transfer of heat from the skillet to the
skillet handle occurs by conduction. But in metals, the conduction mechanism is
slightly more complicated. In a manner similar to electrical conductivity,
thermal conductivity in metals occurs by the movement of free electrons. Outer shell
electrons of metal atoms are shared among atoms and are free to move throughout
the bulk of the metal. These electrons carry the energy from the skillet to the
skillet handle. The details of this mechanism of thermal conduction in metals
are considerably more complex than the discussion given here. The main point to
grasp is that heat transfer through metals occurs without any movement of atoms
from the skillet to the skillet handle. This qualifies the heat transfer as
being categorized as thermal conduction.
Heat
Transfer by Convection
 Is conduction the only means of heat transfer? Can heat be transferred
through the bulk of an object in methods other than conduction? The answer is
yes. The model of heat transfer through the ceramic coffee mug and the metal
skillet involved conduction. The ceramic of the coffee mug and the metal of the
skillet are both solids. Heat transfer through solids occurs by conduction.
This is primarily due to the fact that solids have orderly arrangements of
particles that are fixed in place. Liquids and gases are not very good
conductors of heat. In fact, they are considered good thermal insulators. Heat
typically does not flow through liquids and gases by means of conduction.
Liquids and gases are fluids; their particles are not fixed in place; they move
about the bulk of the sample of matter. The model used for explaining heat
transfer through the bulk of liquids and gases involves convection. Convection is the process of heat transfer from one
location to the next by the movement of fluids. The moving fluid carries energy
with it. The fluid flows from a high temperature location to a low temperature
location.
Is conduction the only means of heat transfer? Can heat be transferred
through the bulk of an object in methods other than conduction? The answer is
yes. The model of heat transfer through the ceramic coffee mug and the metal
skillet involved conduction. The ceramic of the coffee mug and the metal of the
skillet are both solids. Heat transfer through solids occurs by conduction.
This is primarily due to the fact that solids have orderly arrangements of
particles that are fixed in place. Liquids and gases are not very good
conductors of heat. In fact, they are considered good thermal insulators. Heat
typically does not flow through liquids and gases by means of conduction.
Liquids and gases are fluids; their particles are not fixed in place; they move
about the bulk of the sample of matter. The model used for explaining heat
transfer through the bulk of liquids and gases involves convection. Convection is the process of heat transfer from one
location to the next by the movement of fluids. The moving fluid carries energy
with it. The fluid flows from a high temperature location to a low temperature
location.
To understand convection in fluids, let's consider the heat
transfer through the water that is being heated in a pot on a stove. Of course
the source of the heat is the stove burner. The metal pot that holds the water
is heated by the stove burner. As the metal becomes hot, it begins to conduct
heat to the water. The water at the boundary with the metal pan becomes hot.
Fluids expand when heated and become less dense. So as the water at the bottom
of the pot becomes hot, its density decreases. Differences in water density
between the bottom of the pot and the top of the pot results in the gradual
formation of circulation
currents. Hot water begins to rise to the top of the pot displacing the
colder water that was originally there. And the colder water that was present
at the top of the pot moves towards the bottom of the pot where it is heated
and begins to rise. These circulation currents slowly develop over time,
providing the pathway for heated water to transfer energy from the bottom of
the pot to the surface.
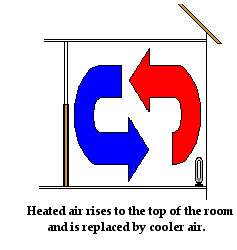 Convection also explains how an electric heater placed on the floor of a
cold room warms up the air in the room. Air present near the coils of the heater
warm up. As the air warms up, it expands, becomes less dense and begins to
rise. As the hot air rises, it pushes some of the cold air near the top of the
room out of the way. The cold air moves towards the bottom of the room to
replace the hot air that has risen. As the colder air approaches the heater at
the bottom of the room, it becomes warmed by the heater and begins to rise.
Once more, convection currents are slowly formed. Air travels along these
pathways, carrying energy with it from the heater throughout the room.
Convection also explains how an electric heater placed on the floor of a
cold room warms up the air in the room. Air present near the coils of the heater
warm up. As the air warms up, it expands, becomes less dense and begins to
rise. As the hot air rises, it pushes some of the cold air near the top of the
room out of the way. The cold air moves towards the bottom of the room to
replace the hot air that has risen. As the colder air approaches the heater at
the bottom of the room, it becomes warmed by the heater and begins to rise.
Once more, convection currents are slowly formed. Air travels along these
pathways, carrying energy with it from the heater throughout the room.
Convection is the main method of heat transfer in fluids such
as water and air. It is often said that heat
rises in these situations.
The more appropriate explanation is to say that heated fluid rises. For
instance, as the heated air rises from the heater on a floor, it carries more
energetic particles with it. As the more energetic particles of the heated air
mix with the cooler air near the ceiling, the average kinetic energy of the air
near the top of the room increases. This increase in the average kinetic energy
corresponds to an increase in temperature. The net result of the rising hot
fluid is the transfer of heat from one location to another location. The
convection method of heat transfer always involves the transfer of heat by the
movement of matter. This is not to be confused with the caloric theory discussed
earlier in this lesson. In caloric theory, heat was the fluid and the fluid
that moved was the heat. Our model of convection considers heat to be energy
transfer that is simply the result of the movement of more energetic particles.
The two examples of convection discussed here - heating water
in a pot and heating air in a room - are examples of natural convection. The driving force of the circulation of fluid is natural
- differences in density between two locations as the result of fluid being heated
at some source. (Some sources introduce the concept of buoyant forces to
explain why the heated fluids rise. We will not pursue such explanations here.)
Natural convection is common in nature. The earth's oceans and atmosphere are
heated by natural convection. In contrast to natural convection, forced convection involves fluid being forced from one location
to another by fans, pumps and other devices. Many home heating systems involve
force air heating. Air is heated at a furnace and blown by fans through
ductwork and released into rooms at vent locations. This is an example of
forced convection. The movement of the fluid from the hot location (near the
furnace) to the cool location (the rooms throughout the house) is driven or
forced by a fan. Some ovens are forced convection ovens; they have fans that
blow heated air from a heat source into the oven. Some fireplaces enhance the
heating ability of the fire by blowing heated air from the fireplace unit into
the adjacent room. This is another example of forced convection.
Heat Transfer by Radiation
A final method of heat transfer involves radiation. Radiation
is the transfer of heat by means ofelectromagnetic waves. To radiate means to send out or spread from a
central location. Whether it is light, sound, waves, rays, flower petals, wheel
spokes or pain, if something radiates then it protrudes or spreads outward
from an origin. The transfer of heat by radiation involves the carrying of
energy from an origin to the space surrounding it. The energy is carried by
electromagnetic waves and does not involve the movement or the interaction of
matter. Thermal radiation can occur through matter or through a region of space
that is void of matter (i.e., a vacuum). In fact, the heat received on Earth
from the sun is the result of electromagnetic waves traveling through the void of space between the Earth and the sun.
All objects radiate energy in the form of electromagnetic
waves. The rate at which this energy is released is proportional to the Kelvin
temperature (T) raised to the fourth power.
Radiation rate = k•T4
The hotter the object, the more it radiates. The sun
obviously radiates off more energy than a hot mug of coffee. The temperature
also affects the wavelength and frequency of the radiated waves. Objects at
typical room temperatures radiate energy as infrared waves. Being invisible to
the human eye, we do not see this form of radiation. An infrared camera is
capable of detecting such radiation. Perhaps you have seen thermal photographs
or videos of the radiation surrounding a person or animal or a hot mug of
coffee or the Earth. The energy radiated from an object is usually a collection
or range of wavelengths. This is usually referred to as an emission spectrum. As the
temperature of an object increases, the wavelengths within the spectra of the
emitted radiation also decrease. Hotter objects tend to emit shorter
wavelength, higher frequency radiation. The coils of an electric toaster are
considerably hotter than room temperature and emit electromagnetic radiation in
the visible spectrum. Fortunately, this provides a convenient warning to its
users that the coils are hot. The tungsten filament of an incandescent light
bulb emits electromagnetic radiation in the visible (and beyond) range. This
radiation not only allows us to see, it also warms the glass bulb that contains
the filament. Put your hand near the bulb (without touching it) and you will
feel the radiation from the bulb as well.

Thermal radiation is a form of heat transfer because the electromagnetic
radiation emitted from the source carries energy away from the source to
surrounding (or distant) objects. This energy is absorbed by those objects,
causing the average kinetic energy of their particles to increase and causing
the temperatures to rise. In this sense, energy is transferred from one location
to another by means of electromagnetic radiation. The image at the right was
taken by a thermal imaging camera. The camera detects the radiation emitted by
objects and represents it by means of a color photograph. The hotter colors represent areas of objects that are
emitting thermal radiation at a more intense rate. (Images courtesy Peter Lewis
and Chris West of Standford's SLAC.)
Our discussion on this page has pertained to the various
methods of heat transfer. Conduction, convection and radiation have been
described and illustrated. The macroscopic has been explained in terms of the
particulate - an ongoing goal of this chapter of The Physics Classroom
Tutorial. The last topic to be discussed in Lesson 1 is more quantitative in
nature. On the next page, we will investigate the mathematics
associated with the rate of heat transfer.
Check Your Understanding
1. Consider Object A which has a temperature of 65°C and
Object B which has a temperature of 15°C. The two objects are placed next to
each other and the little
bangers begin colliding. Will
any of the collisions result in the transfer of energy from Object B to Object
A? Explain.

Answer: Most certainly yes.
The average
kinetic energy of the particles in Object A is greater than the average kinetic
energy of the particles in Object B. But there is a range of speeds and thus of
kinetic energy in both objects. As such, there will be some highly energetic
particles in Object B and some very non-energetic particles in Object A. When
this combination of particles encounter a collision, there will a transfer of
energy across the boundary from Object B (the colder object) to Object A (the hotter
object). This is just one collision. Since majority of collisions result from
the more energetic particles of Object A with less energetic particles of
collision B, there will be a net kinetic energy transfer from Object A to
Object B.
2. Suppose that Object A and Object B (from the previous
problem) have reached a thermal equilibrium. Do the particles of the two
objects still collide with each other? If so, do any of the collisions result
in the transfer of energy between the two objects? Explain.

Answer:
The collisions
will still take place because the particles are still moving. Just because the
temperatures are the same doesn't mean the collisions will stop. The fact that
the temperature is identical means that the average kinetic energy of all the
particles is the same for both objects. As such, there will be just as much
energy transferred from Object B to Object A
as there is energy transferred in the opposite direction. When the effect of
these collisions is averaged, there is no net energy transfer. This
explains why the temperature of the two objects remains the same. Thermal
equilibrium persists.