Thermometers as Speedometers
On the previous page of this
lesson, temperature was defined as the reading on a thermometer. The process of
calibrating a thermometer was explained and the variety of commonly used
temperature scales were described. Finally, the concept of an absolute lowest
temperature was discussed. But in the end, the fundamental definition of
temperature was not given. Temperature was only defined in practical terms -
the reading on a thermometer. Now we have to answer the more fundamental
question: what is the reading on a thermometer the reflection of? What is
temperature a measure of?
 Temperature as
a Measure of Kinetic Energy
Temperature as
a Measure of Kinetic Energy
It is at this point that we can use a more sophisticated
definition of temperature. Temperature is a measure of the average kinetic
energy of the particles within a sample of matter. In a previous
unit of The Physics Classroom Tutorial, kinetic
energy was defined as the energy of motion. An object ... or a particle ...
that is moving has kinetic energy. There are three common forms of kinetic
energy - vibrational kinetic energy, rotational kinetic energy and
translational kinetic energy. Up to this point of the Tutorial, we have
associated kinetic energy with the movement of an object (or particle) from one
location to another. This is referred to as translational kinetic energy. A
ball moving through space has translational kinetic energy. But an object
can also have vibrational kinetic energy; this is the energy of motion
possessed by an object that is oscillating or vibrating about a fixed position.
And a mass attached to a spring has vibrational kinetic energy. Such a mass is
not permanently displaced from its position like a ball moving through space.
Finally an object can have rotational kinetic energy; this is the energy
associated with an object that is rotating about an imaginary axis of rotation.
A spinning top isn't moving through space and isn't
vibrating about a fixed position, but there is still kinetic energy associated
with its motion about an axis of rotation. This form of kinetic energy is
called rotational kinetic energy.
A sample of matter consists of particles that can be
vibrating, rotating and moving through the space of its container. So at the
particle level, a sample of matter possesses kinetic energy. A warm cup of
water on a countertop may appear to be as still as can be; yet the particles
that are contained within it have kinetic energy. At the particle level, there
are atoms and molecules that are vibrating, rotating and moving through the
space of its container. Stick a thermometer in the cup of water and you will
see the evidence that the water possesses kinetic energy. The water's
temperature, as reflected by the thermometer's reading, is a measure of the
average amount of kinetic energy possessed by the water molecules.
 When the temperature of an object increases, the particles that compose
the object begin to move faster. They either vibrate more rapidly, rotate with
greater frequency or move through space with a greater speed. Increasing the
temperature causes an increase in the particle speed. So as a sample of water
in a pot is heated, its molecules begin to move with greater
speed and this greater speed is reflected by a higher thermometer reading.
Similarly, if a sample of water is placed in the freezer, its molecules begin to move slower (with a lower speed) and this is
reflected by a lower thermometer reading. It is in this sense that a
thermometer can be thought of as a speedometer.
When the temperature of an object increases, the particles that compose
the object begin to move faster. They either vibrate more rapidly, rotate with
greater frequency or move through space with a greater speed. Increasing the
temperature causes an increase in the particle speed. So as a sample of water
in a pot is heated, its molecules begin to move with greater
speed and this greater speed is reflected by a higher thermometer reading.
Similarly, if a sample of water is placed in the freezer, its molecules begin to move slower (with a lower speed) and this is
reflected by a lower thermometer reading. It is in this sense that a
thermometer can be thought of as a speedometer.
Boltzmann
Speed Distribution and Average Kinetic Energy
At the onset of this page, temperature was defined as a
measure of the average amount of kinetic energy possessed by an object. But
what exactly is meant by average kinetic
energy? In any sample of matter, particles are moving. Consider the sample of
helium gas inside of a helium-filled balloon. The predominant motion of the
helium atoms is translational motion. The helium atoms move through the space
of the balloon from one location to another. As they do, they encounter
collisions with one another and with the balloon walls. These collisions result
in changes in speed and direction. As a result, there is not a single speed at
which the helium atoms move, but a range of speeds. Being that there is a range
of speeds with which the helium atoms move, there is a range of kinetic
energies possessed by these particles. This is often referred to as a Boltzmann
speed distribution and is represented graphically by the diagram below. We will
return to discuss this topic in the next chapter of The Physics Classroom
Tutorial.
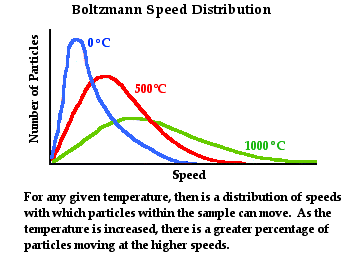
If you've been following through this lesson from the
beginning, then you understanding of temperature is becoming increasingly
sophisticated. You now know that the temperature is more than what the
thermometer reads; it is a reflection of the average kinetic energy with which
the particles move. The macroscopic description of matter - a thermometer
reading - is tied to a particulate description of matter - the speed with which
particles move. Now we have to probe the question: what is the relationship
between temperature and heat? What is heat? Is temperature the same thing as
heat? Is temperature in any way related to heat? What is the cause of heat?
These are the questions that we will ponder in the next
section of Lesson 1.
Check Your Understanding
1. Consider two samples of different gases. One sample
consists of helium atoms and the other sample consists of diatomic oxygen
molecules. If the samples are at the same temperature, will the particles
within the sample have the same average speed?

Answer: No
Temperature is a
measure of the average kinetic energy of the samples. Translational kinetic
energy depends upon both the mass of the particles and the average speed at
which the particles move. In comparing two samples of different gases at the
same temperature, the gas with the more massive particles has the slowest
particle speeds. So in comparing the speeds of helium atoms and diatomic oxygen
molecules, one must be conscious of the relative masses of the two particles.
Helium particles, being roughly one-eighth the mass of diatomic oxygen
molecules, will move with a considerably faster speed.
2. The particles in a sample of table salt (sodium chloride)
are not free to move about. They are locked in place in a structure known as a
crystal lattice. Can the particles of sodium chloride possess kinetic energy?

Answer: Yes
Even though they
do no possess any translational kinetic
energy, they still possess some vibrational kinetic energy. The sodium and
chloride ions can wiggle about their fixed lattice positions. The back and
forth vibrational motion of the particles is what gives them vibrational
kinetic energy. This explains why a thermometer will register a temperature
when placed in the sample of matter.