Conductors and Insulators
The behavior of an object that has been charged is dependent upon whether the
object is made of a conductive or a nonconductive material. Conductors are
materials that permit electrons to flow freely from particle to particle. An
object made of a conducting material will permit charge to be transferred
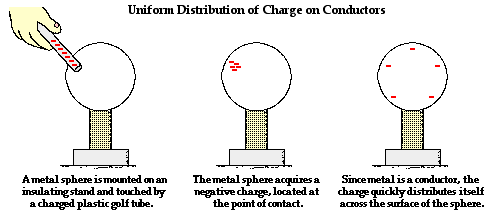
across the entire surface of the object. If charge is transferred to the object
at a given location, that charge is quickly distributed across the entire
surface of the object. The distribution of charge is the result of electron
movement. Since conductors allow for electrons to be transported from particle
to particle, a charged object will always distribute its charge until the
overall repulsive forces between excess electrons is minimized. If a charged
conductor is touched to another object, the conductor can even transfer its
charge to that object. The transfer of charge between objects occurs more readily
if the second object is made of a conducting material. Conductors allow for
charge transfer through the free movement of electrons.
In contrast to conductors, insulators are materials that impede the free flow of
electrons from atom to atom and molecule to molecule. If charge is transferred
to an insulator at a given location, the excess charge will remain at the
initial location of charging. The particles of the insulator do not permit the
free flow of electrons; subsequently charge is seldom distributed evenly across
the surface of an insulator.
While insulators are not useful for transferring charge, they
do serve a critical role in electrostatic experiments and demonstrations.
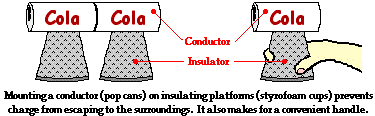
Conductive objects are often mounted upon insulating objects. This arrangement
of a conductor on top of an insulator prevents charge from being transferred
from the conductive object to its surroundings. This arrangement also allows
for a student (or teacher) to manipulate a conducting object without touching
it. The insulator serves as a handle for moving the conductor around on top of
a lab table. If charging experiments are performed with aluminum pop
cans, then the cans should be mounted on top of Styrofoam cups. The cups serve
as insulators, preventing the pop cans from discharging their charge. The cups
also serve as handles when it becomes necessary to move the cans around on the
table.
Examples
of Conductors and Insulators
Examples of conductors include metals, aqueous solutions of
salts (i.e., ionic compounds dissolved in water), graphite, and the
human body. Examples of insulators include plastics, Styrofoam, paper, rubber,
glass and dry air. The division of materials into the categories of conductors
and insulators is a somewhat artificial division. It is more appropriate to
think of materials as being placed somewhere along a continuum. Those materials
that are super conductive (known as superconductors) would be placed at on end and the
least conductive materials (best insulators) would be placed at the other end.
Metals would be placed near the most conductive end and glass would be placed
on the opposite end of the continuum. The conductivity of a metal might be as
much as a million trillion times greater than that of glass.

Along the continuum of conductors and insulators, one might
find the human body somewhere towards the conducting side of the middle. When the body acquires
a static charge it has a tendency to distribute that charge throughout the
surface of the body. Given the size of the human body, relative to the size of
typical objects used in electrostatic experiments, it would require an
abnormally large quantity of excess charge before its effect is noticeable. The
effects of excess charge on the body are often demonstrated using a Van
de Graaff generator. When a student places their hand upon the static ball,
excess charge from the ball is shared with the human body. Being a conductor,
the excess charge could flow to the human body and spread throughout the
surface of the body, even onto strands of hair. As the individual strands of
hair become charged, they begin to repel each other. Looking to distance
themselves from their like-charged neighbors, the strands of hair begin to rise upward and
outward - a truly hair-raising experience.
Many are familiar with the impact that humidity can have upon
static charge buildups. You have likely noticed that bad hair days, doorknob shocks and static
clothing are most common during winter months. Winter months tend to be the
driest months of the year with humidity levels in the air dropping to lower
values. Water has a tendency to gradually remove excess charge from objects.
When the humidity is high, a person acquiring an excess charge will tend to
lose that charge to water molecules in the surrounding air. On the other hand,
dry air conditions are more conducive to the buildup of static charge and more frequent electric
shocks. Since humidity levels tend to vary from day to day and season to
season, it is expected that electrical effects (and even the success of
electrostatic demonstrations) can vary from day to day.
Distribution
of Charge via Electron Movement
Predicting the direction that electrons would move within a
conducting material is a simple application of the two fundamental rules of
charge interaction. Opposites attract and likes repel. Suppose that some method
is used to impart a negative charge to an object at a given location. At the
location where the charge is imparted, there is an excess of electrons. That
is, the multitude of atoms in that region possess more electrons than protons.
Of course, there are a number of electrons that could be thought of as beingquite contented since there is an accompanying positively
charged proton to satisfy their attraction for an opposite. However, the
so-called excess electrons have a repulsive response to each other and would
prefer more space. Electrons, like human beings, wish to manipulate their
surroundings in an effort to reduce repulsive effects. Since these excess
electrons are present in a conductor, there is little hindrance to their
ability to migrate to other parts of the object. And that is exactly what they
do. In an effort to reduce the overall repulsive effects within the object,
there is a mass migration of excess electrons throughout the entire surface of
the object. Excess electrons migrate to distance themselves from their
repulsive neighbors. In this sense, it is said that excess negative charge distributes
itself throughout the surface of the conductor.
But what happens if the conductor acquires an
excess of positive charge? What if electrons are removed from a conductor at a
given location, giving the object an overall positive charge? If protons cannot
move, then how can the excess of positive charge distribute itself across the
surface of the material? While the answers to these questions are not as
obvious, it still involves a rather simple explanation that once again relies
on the two fundamental rules of charge interaction. Opposites attract and likes
repel. Suppose that a conducting metal sphere is charged on its left side and
imparted an excess of positive charge. (Of course, this requires that electrons
be removed from the object at the location of charging.) A multitude of atoms
in the region where the charging occurs have lost one or more electrons and
have an excess of protons. The imbalance of charge within these atoms creates
effects that can be thought of as disturbing the balance of charge within the
entire object. The presence of these excess protons in a given location draws
electrons from other atoms. Electrons in other parts of the object can be
thought of as being quite
contented with the balance of
charge that they are experiencing. Yet there will always be some electrons that
will feel the attraction for the excess protons some distance away. In human
terms, we might say these electrons are drawn by curiosity or by the belief
that the grass is greener on the other side of the fence. In the language of
electrostatics, we simply assert that opposites attract - the excess protons
and both the neighboring and distant electrons attract each other. The protons cannot do
anything about this attraction since they are bound within the nucleus of their
own atoms. Yet, electrons are loosely bound within atoms; and being present in
a conductor, they are free to move. These electrons make the move for the
excess protons, leaving their own atoms with their own excess of positive
charge. This electron migration happens across the entire surface of the
object, until the overall sum of repulsive effects between electrons across the
whole surface of the object are minimized.
Check Your Understanding
Use your understanding of charge to answer the following
questions. When finished, click the button to view the answers.
1. One of these isolated charged spheres is copper and the
other is rubber. The diagram below depicts the distribution of excess negative
charge over the surface of two spheres. Label which is which and support your
answer with an explanation.
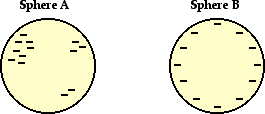

Answer: A is rubber and B is copper.
Sphere A shown a
non-uniform distribution of excess charge; so sphere A must be made of an
insulating material such as rubber. Sphere B shows a uniform distribution of
excess charge; one would reason that it is made of a conductor such as copper.
close
2. Which of the following materials are likely to exhibit
more conductive properties than insulating properties? _____ Explain your
answers.
|
a. rubber |
b. aluminum |
c. silver |
d. plastic |
e. wet
skin |

Answer; B, C and E
Aluminum and silver are
metals, making them good conductors. The human body is a fairly good conductor.
When wet, its an even better conductor.
3. A conductor differs from an insulator in that a conductor
________.
a. has an excess
of protons
b. has an excess
of electrons
c. can become
charged and an insulator cannot
d. has faster
moving molecules
e. does not have any
neutrons to get in the way of electron flow
f. none of these

Answer: F
A
and B
are characteristic of positive and negative objects. As for C, both insulators
and conductors can be charged. As for D, this has nothing to do with the
conductive properties of materials. As for E, neutrons are located in the
nucleus and are "out of the way" of mobile electrons.
4. Suppose that a conducting sphere is charged positively by
some method. The charge is initially deposited on the left side of the sphere.
Yet because the object is conductive, the charge spreads uniformly throughout
the surface of the sphere. The uniform distribution of charge is explained by
the fact that ____.
a. the charged
atoms at the location of charge move throughout the surface of the sphere
b. the excess
protons move from the location of charge to the rest of the sphere
c. excess
electrons from the rest of the sphere are attracted towards the excess protons

Answer: C
Rule out A since
atoms are not capable of moving within solid spheres. Rule out B since protons
are not capable of moving in electrostatic demos. C is the proper explanation
since the negative electrons are attracted to the region of positive charge.
The electrons migrate towards the left side of the sphere until there is a
uniform distribution of positive charge.
5. When an oil tanker car has arrived at its destination, it
prepares to empty its fuel into a reservoir or tank. Part of the preparation
involves connecting the body of the tanker car with a metal wire to the ground.
Suggest a reason for why is this done.

As fuel is
pumped from the tanker car to a reservoir, charge can quickly build up as the
fluid flows through the hoses. This static charge can create sparks capable of
igniting the fuel. By connecting the body of the tanker car to the ground, the
static charge can be transferred to the ground. A metal wire is used since
metals are conductive and allow charge to flow through them.