Division and Germer’s experiment:
The first experimental evidence of the wave nature of atomic particles was proved by C.J Division and L.H Germer in 1927. They were studying scattering of electrons by a metal target and measuring the density of electrons scattered in different directions.
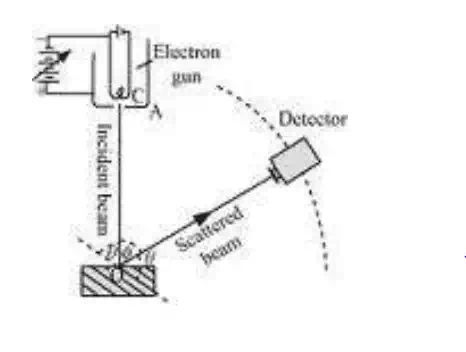
1. From fig, the electron beam from electron gun which consists of a tungsten filament „F‟ heated by a low tension battery „B1‟ are accelerated to a desired velocity by applying suitable potential from a high tension battery „B2‟.
2. The accelerated electrons are collimated into a fine beam by allowing them to pass thorough a system of pinholes in the cylinder „C‟.
3. The fast moving electron beam is made to strike the target (nickel crystal) capable of rotating about an axis perpendicular to the plane of diagram.
4. The electrons are scattered in all directions by atomic planes of a crystal and intensity of scattered electron beam in all directions can be measured by the electron collector and can be rotated about the same axis as the target.
5. The collector is connected to a sensitive galvanometer whose deflection is proportional to the intensity of electron beam entering the collector.
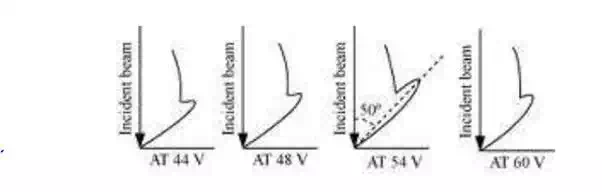
6. When electron beam accelerated by 54 V was directed to strike the given nickel crystal, a sharp max in the electron diffraction occurred at an angle of 500 with the incident beam.
7. The incident beam and the diffracted beam make an angle of 650 with the family of Bragg’s planes.
8. The whole instrument is kept in an evacuated chamber.
9. The spacing of planes in Nickel crystal as determined by x-ray diffraction is 0.091nm
From Bragg’s law
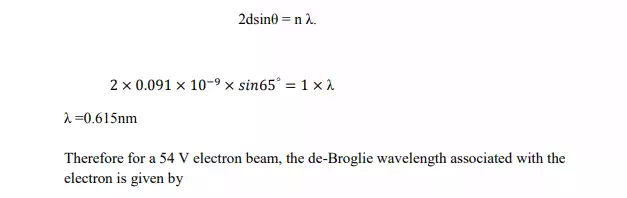
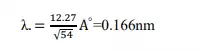
This wavelength agrees well with the experimental value. Thus division experiment provides a direct verification of de-Broglie hypothesis of wave nature of moving particles.