Defects In Crystals
In a perfect crystal:
1. Atoms exist only in lattice sites.
2. Periodicity of lattice extends up to infinity.
3. Atoms do not loose electrons; no free electrons are present.
4. Atoms would be stationary.
Any deviation from the above four properties is known as defect.
The properties of crystal are divided into two categories based on the presence of imperfections.
1. Structure sensitive properties: which are affected by the presence of imperfections. Ex: mechanical strength, electrical conductivity in semiconductors, magnetic hysteresis, dielectric strength.
2. Structure insensitive properties: which are not affected by the presence of imperfections. Ex: stiffness.
Defects are produced by various methods:
1. by heating
2. Rapid cooling
3. By applying external strength
4. By bombarding with high energy particles like neutrons or particles from cyclotron
Point defects: (zero dimensional defects) arises when an atom is absent from the regular position, presence of impurity atom or atom in the wrong place during crystallization.. These are small defects which extends its influence in all directions but limited to a specific region of small order (two or three atomic orders).
Point defects are:
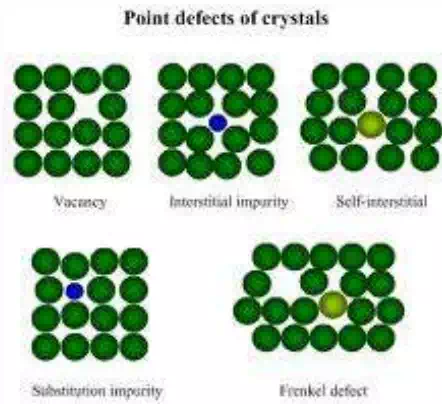
Vacancy: missing of an atom from its original lattice site. Generally arises due to thermal vibrations during crystallization and influenced by external parameters. Vacancies may be single, two or more depending on crystal type. For most of the crystals, in order to create one vacancy thermal energy of 1.1 ev is required.
Interstitial: this defect arises when an atom of same kind or different kind occupies the void space between the regular atomic sites.
Impurity atom: an atom that does not belong to the parent lattice (original crystal).
Substitutional defects: this defect arises when an impurity atom replaces or substitutes parent atom.
Ex: in brass, zinc is a
substitutional atom in a copper lattice
Interstitial impurity: this defect arises when an
impurity atom which is small in size is placed between the regular atomic
sites.
Ex: when pentavalent and trivalent impurities are added to pure Si or Ge, we get n-type and P-type semiconductors.
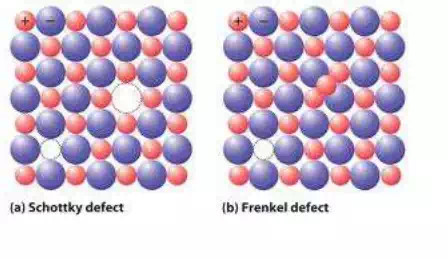
Imperfections in ionic crystals: in case of ionic crystals imperfections appear in crystals while maintaining the electrical neutrality. two types of defects (point defects) occur in ionic crystals.
1. Frenkel defect
2. Schottky defect.
Frenkel defect: When an ion is displaced from a regular lattice site to an interstitial site is called Frenkel defect. Generally cations which are small in size are displaced to an interstitial site as the interstitial space is small.
A Frenkel imperfection does not change the overall electrical neutrality of the crystal.
Schottky defect: A pair of one cation and one anion missing from the original lattice site on to the surface of the crystal so that charge neutrality is maintained in the crystal is called Schottky defect.
Line defects (or) dislocations (one dimensional defect) dislocation is defined as the disturbed region between the two perfect parts of the crystal and these defects are formed in the process of deformation.
Edge dislocation: A perfect crystal is composed of several parallel vertical planes which are extended from top to bottom completely and parallel to side faces. The atoms are in equilibrium positions and the bond lengths are in equilibrium value.
If one of the vertical planes does not extend from top to bottom face of the crystal, but ends in midway within the crystal, then crystal suffers with a dislocation called edge dislocation.
In imperfect crystal all the atoms above the dislocation plane are squeezed together and compressed there by the bond length decreases. And all the atoms below the dislocation plane are elongated by subjecting to the tension and thereby the bond length increases. There are two types of edge dislocation.
They are
1. Positive edge dislocation
2. Negative edge dislocation.
Positive edge dislocation: if the vertical plane starts from top of the crystal and never reaches to the bottom .
Negative edge dislocation: if the vertical plane starts from bottom of the crystal and never reaches top
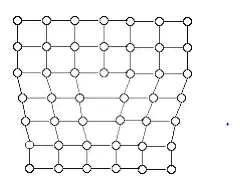
Screw dislocation; atoms are displaced in two separate planes perpendicular to each other or defects forming a spiral around the dislocation line.
A screw dislocation marks the boundary between slipped and unslipped parts of the crystal, that can be produced by cutting the crystal partway and then sheering down one part relative to the other by atomic spacing horizontally