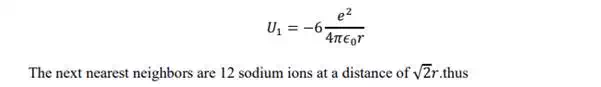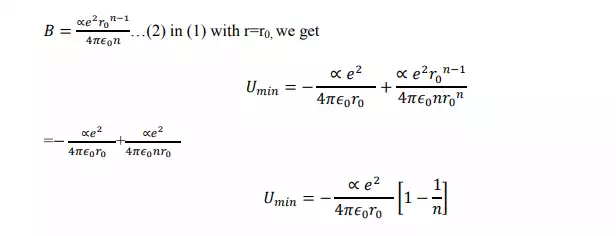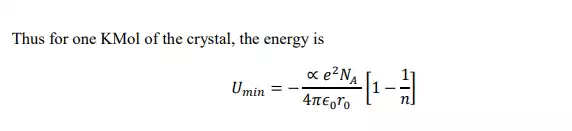Calculation of Lattice energy of Ionic crystals:
The lattice energy of an ionic solid will differ from the bond energy of diatomic soilds.in the former case there will be interactions between more atoms.
“The cohesive energy of an ionic crystal is the energy that would be liberated by the formation of the crystal from individual neutral atoms
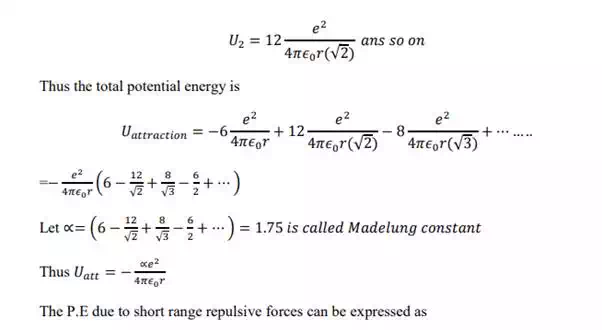
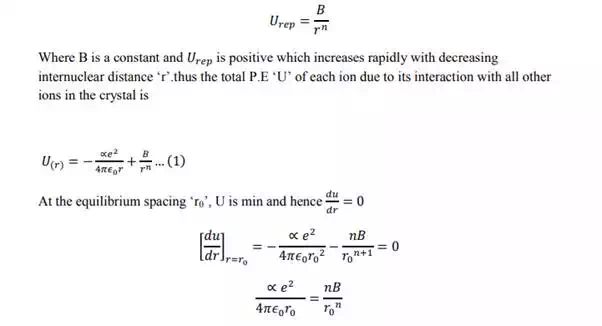
In case of NaCl crystal, each sodium ion is subjected to attractive potential due to 6 chloride ions each at a distance „r‟. thus the attractive potential at the sodium ion by the chloride ion is