Semiconductors
Energy bond Formation in solids:
- In isolated atom, the eˉs are tightly bond and have discrete, shape energy levels.
- When two identical atoms are brought closed the outermost orbits of these atoms overlap and intersects the wave functions of the E of the different atoms begin to overlap, then the energy levels corresponding to those wave functions split in to two.


- If more atoms brought together more levels are formed and for a solid of N atoms, each of these energy levels of an atoms splits into N levels of energy.
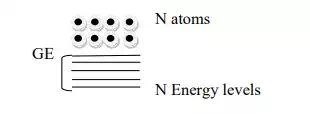
The levels are so close together that they form almost continuous bond.
-The E first occupies lower energy bonds and are no importance in determining many of the physical properties of solid.
-These E present in higher energy bonds are important in determining many of the physical of solids.
-These two allowed energy bonds are called as valence and conduction bonds.
-The bond corresponding to the outermost orbit is called conduction bond and the gap between those two allowed bonds is called forbidden energy gap are bond gap.
Classification of solids:
Solids are classified into three types based on energy gap.
(1) Conductors(metal)
(2) Insulators
(3) Semiconductors
-In case of conductors, valence bond and conduction bond almost overlap each other and no significance in energy gap. The two allowed bond are separated by semi energy level.
-Here there is no role in Eg, as a result conducting is high.
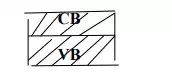
-In case insulator, valence bond and conduction bond are separated by large energy gap, Hence conductivity is zero.
-In case semiconductors, the valence bond and conduction bond are separated by relatively narrow energy gap, hence the conductivity lies in between conductors and insulators.
Effective mass of the E moving in a crystal lattice:
-Consider a crystal (metal) be subjected to an electric field „E‟, so the experienced by an E of charge E is Ee.
-Acceleration of the E in the crystal is given by a=f/m=Ee/m
-But acceleration of the E is not constant because of the velocity changes i.e The E move faster near the +ve ions in the crystal. Since the electric field and charge of the E are invariant, the effective mass Mh of the E to change accordingly.
i.e F=mha (1)
-Consider a particle velocity „v‟ is equal to group velocity „vg‟ of a wave packet, then