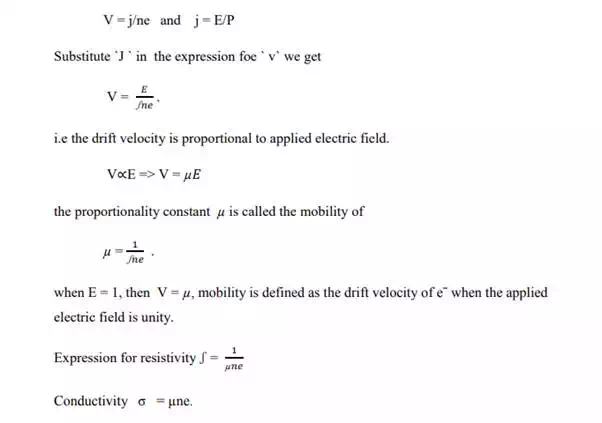
Relation Between Drift Velocity And Mobility Of The Electron:-
According to the ohm`s law R = v/i , where `v` is the potential difference between the ends of a conductor, `i` is the current flowing though the conductor and `R` is the resistance.
The ohm`s law can otherwise be stated as E/J = P called microscopic ohm`s law . here `E` is the electric filed, `J` is the current density flowing through the conductor and `p` is the resistivity or specific resistance of the metal
Failure Of The Classical Free Electron Theory:-
1) Heat capacities :- the internal energy of a molar substances
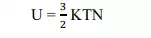
Molar specific heat + c v =3/2kn=3/2R. „
N‟ is the Alvarado number , K is Boltzmann constant and `R` is the universal gas constant .the molar specific heat is 1.5 R theoretically whereas the experimental value obtained is too low . This is due to the fact that all free electrons do not contribute significantly to thermal or electrical conductivity.
Therefore classical free eˉ theory can`t hold good.
2) Mean free path :- It is calculated using the formula
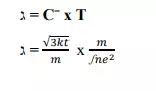
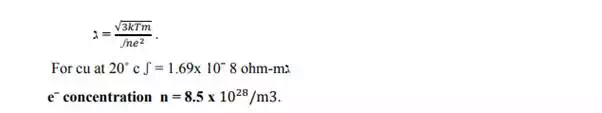
3) RESISTIVITY:- According to the classical free electron theory ,the resistivity is given by the equation.
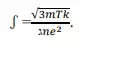
Which means the
resistivity is proportional to the square root of absolute temperature. But
according to the at room temperature it does not change up to 10K and in
intermediate range of temperature is proportional to 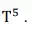
4) The conductivity of semiconductors and Insulators cannot be explained by the free electron theory.
Quantum Free Electron Theory:-
In 1929, Somerfield stated to apply quantum mechanics to explain conductivity phenomenon in metal. He has improved the Drude ƹ Lorentz theory by quantizing the free electron energy and retaining the classical concept of free motion of electron at a random .
ASSUMPTIONS:-
1) The electrons are free to move within the metal like gaseous molecules. They are confined to the metal due to surface potential.
2) The velocity distribution of the free electrons is described by Fermi-Divac Statistics because electrons are spin half particles.
3) The free electrons would be go into the different energy levels by following Pauli‟s exclusion Principle which states that no two electrons have same set of Quantum numbers.
4) The motion of electrons is associated with a complex wave called matter wave, according to Debroglie hypothesis.
5) The electrons cannot have all energies but will have discrete energies according to the equation .
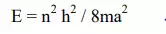
„a‟ is the dimensions of the metal „h‟ is plank‟s constant and „n‟ is the integer starts from 1.