|
The Simplest Repeating Unit in a Crystal |
A Three-Dimensional Graph |
|
NaCl and ZnS |
Measuring the Distance Between Particles |
|
Determining the Unit Cell of a Crystal |
Calculating Metallic or Ionic Radii |
Unit Cells: The Simplest Repeating Unit in a Crystal
The structure of solids can be described as if they were three-dimensional analogs of a piece of wallpaper. Wallpaper has a regular repeating design that extends from one edge to the other. Crystals have a similar repeating design, but in this case the design extends in three dimensions from one edge of the solid to the other.
We can unambiguously describe a piece of wallpaper by specifying the size, shape, and contents of the simplest repeating unit in the design. We can describe a three-dimensional crystal by specifying the size, shape, and contents of the simplest repeating unit and the way these repeating units stack to form the crystal.
The simplest
repeating unit in a crystal is called a unit cell. Each unit cell
is defined in terms of lattice points the points in space about
which the particles are free to vibrate in a crystal.
the points in space about
which the particles are free to vibrate in a crystal.
The structures of the unit cell for a variety of salts are shown below.

In 1850, Auguste Bravais showed that crystals could be divided into 14 unit cells, which meet the following criteria.
- The unit cell is the simplest repeating unit in the crystal.
- Opposite faces of a unit cell are parallel.
- The edge of the unit cell connects equivalent points.
The 14 Bravais unit cells are shown in the figure below.

These unit cells fall into seven categories, which differ in the three unit-cell edge lengths (a, b, and c) and three internal angles (a, � and g), as shown in the table below.
The Seven Categories of Bravais Unit Cells
|
Category |
|
Edge Lengths |
|
Internal Angles |
|
Cubic |
|
(a = b = c) |
|
(a = �/i> = g = 90o) |
|
Tetragonal |
|
(a = b |
|
(a = �/i> = g = 90o) |
|
Monoclinic |
|
(a |
|
(a = �/i> = 90o |
|
Orthorhombic |
|
(a |
|
(a = �/i> = g = 90o) |
|
Rhombohedral |
|
(a = b = c) |
|
(a = �/i> = g |
|
Hexagonal |
|
(a = b |
|
(a = �/i> = 90o, g = 120o) |
|
Triclinic |
|
(a |
|
(a |
We will
focus on the cubic category, which includes the three types of unit cells simple
cubic, body-centered cubic, and face-centered cubic
simple
cubic, body-centered cubic, and face-centered cubic shown in the figure
below.
shown in the figure
below.
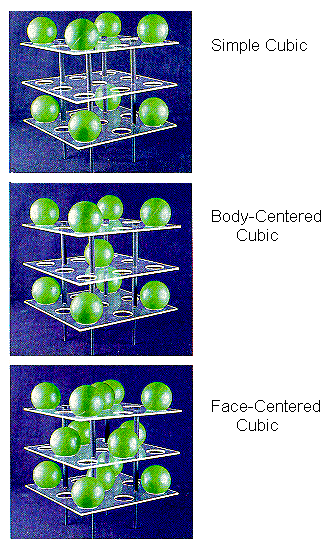
These unit cells are important for two reasons. First, a number of metals, ionic solids, and intermetallic compounds crystallize in cubic unit cells. Second, it is relatively easy to do calculations with these unit cells because the cell-edge lengths are all the same and the cell angles are all 90.
The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. By convention, the edge of a unit cell always connects equivalent points. Each of the eight corners of the unit cell therefore must contain an identical particle. Other particles can be present on the edges or faces of the unit cell, or within the body of the unit cell. But the minimum that must be present for the unit cell to be classified as simple cubic is eight equivalent particles on the eight corners.
The body-centered cubic unit cell is the simplest repeating unit in a body-centered cubic structure. Once again, there are eight identical particles on the eight corners of the unit cell. However, this time there is a ninth identical particle in the center of the body of the unit cell.
The face-centered cubic unit cell also starts with identical particles on the eight corners of the cube. But this structure also contains the same particles in the centers of the six faces of the unit cell, for a total of 14 identical lattice points.
The face-centered cubic unit cell is the simplest repeating unit in a cubic closest-packed structure. In fact, the presence of face-centered cubic unit cells in this structure explains why the structure is known as cubic closest-packed.
 c)
c)