Wet corrosion or electrochemical corrosion
· This type of Corrosion occurs where a conducting liquid is in contact with the metal. This corrosion occurs due to the existence of separate anodic and cathodic parts, between which current flows through the conducting solution.
· At anodic area, oxidation reaction occurs there by destroying the anodic metal either by dissolution or formation of compounds. Hence corrosion always occurs at anodic parts.
Mechanism: Electrochemical corrosion involves flow of electrons between anode and cathode.
The anodic reaction involves dissolution of metal liberating free electrons.

The cathodic reaction consumes electrons with either evolution of hydrogen or absorption of oxygen which depends on the nature of corrosive environment.
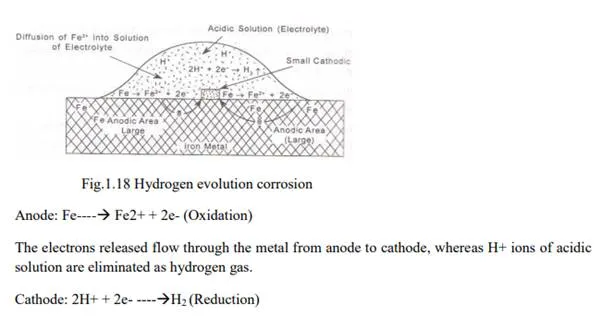
Evolution of hydrogen: This type of corrosion occurs in acidic medium. E.g. Considering the metal Fe, anodic reaction is dissolution of iron as ferrous ions with Liberation of electrons.