Corrosion and Its Control
Corrosion
The surface of almost all the metals begin to decay more or less rapidly when exposed to atmospheric gases, water or other reactive liquid medium.
The process of decay metal by environmental attack is known as corrosion. Metals undergo corrosion and convert to their oxides, hydroxides, carbonates, sulphides etc.
E.g. Iron undergoes corrosion to form reddish brown colour rust [Fe2O3. 3H2O].
Copper undergoes corrosion to form a green film of basic carbonate [CuCO3 + Cu (OH) 2]
Causes of corrosion
1. The metals exist in nature in the form of their minerals or ores, in the stable combined forms as oxides, chlorides, silicates, carbonates, sulphides etc.
2. During the extraction of metals, these ores are reduced to metallic state by supplying considerable amounts of energy.
3. Hence the isolated pure metals are regarded as excited states than their corresponding ores. So metals have natural tendency to go back to their combined state (minerals/ores).
When metal is exposed to atmospheric gases, moisture, liquids etc., and the metal surface reacts and forms more thermodynamically stabled compounds.
Effects of corrosion
1. Wastage of metal in the form of its compounds.
2. The valuable metallic properties like conductivity, malleability, ductility etc. are lost due to corrosion.
3. Life span and efficiency of metallic parts of machinery and fabrications is reduced.
Theories of corrosion
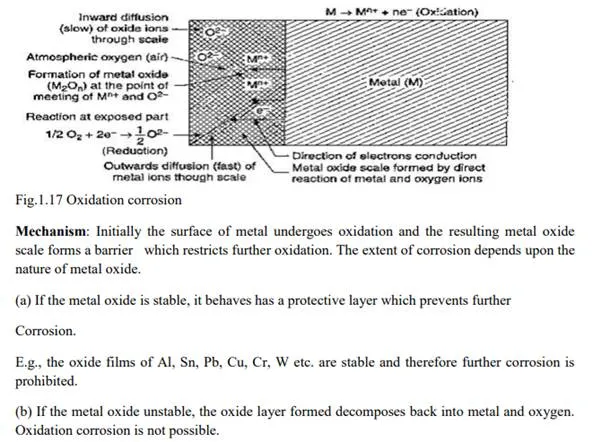
Dry corrosion or Chemical corrosion
This type of Corrosion occurs mainly through the direct chemical action of atmospheric gasses like O2, halogens, H2S, SO2, N2 or anhydrous inorganic liquid with the metal surface.
There are three types of chemical Corrosion: