Reference Electrodes
Because of the inconveniences in the usage of Hydrogen electrode like maintenance of accurate pressure, inconvenience in handling gas secondary electrodes were developed.
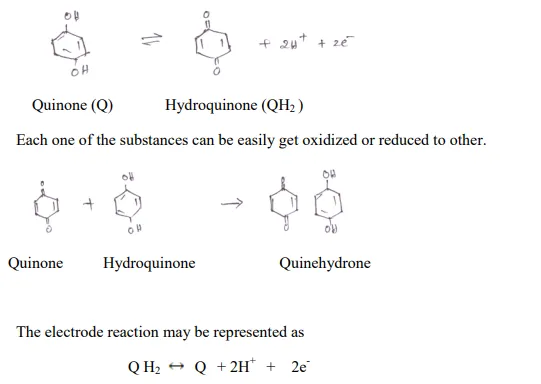
Quinhydrone Electrode
It is a type of redox electrode which can be used to measure H+ concentration of a solution. Quine hydrone is an equimolar (1:1) mixture of quinine and hydroquinone. The electrode consists of pt electrode dipped in an acid or base test solution which is saturated with quine hydrone. The electrode reaction is.



Types of electrodes
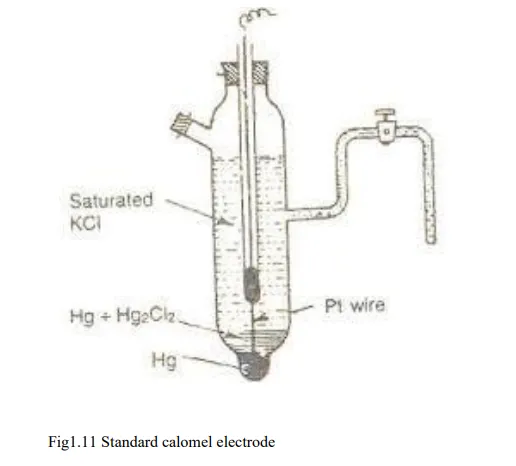
Standard calomel electrode (SCE):-
The calomel electrode consists of a glass tube having two side tubes. A small quantity of pure mercury is placed at the bottom of the vessel and is covered with a paste of Hg and Hg2 Cl2. KCl solution of known concentration is filled through side tube, Shown on the right side of the vessel. The KCl sol. is filled in the left side tube which helps to make a connection through a salt bridge with the other electrode, which potential has to be determined.
A ‘pt’ wire is sealed into a glass tube as shown in the fig which is in contact with Hg.
When the cell is set up it is immersed in the given solution. The concentration of KCl. The electrode potentials of calomel electrode of different concentrations at 25deg c are