Electro chemical cell (or) Galvanic cell
Galvanic cell is a device in which chemical energy is converted into electrical energy. These cells are called Electrochemical cells or voltaic cells. Daniel cell is an example for galvanic cell.

This cell is made up of two half cells. One is oxidation or anodic half-cell. The other is reduction or catholic half-cell. The first half cell consists of ‘Zn’ electrode dipped in ZnSO4solution and second half cell consists of ‘Cu” electrode dipped in Cuso4 solution. Both the half cells are connected externally by metallic conductor. And internally by ‘salt bridge’ salt bridge is a U- tube containing concentrated solution of kCl or NH4 NO3 in agar-agar gel contained porous pot. It provides electrical contact between two solutions.
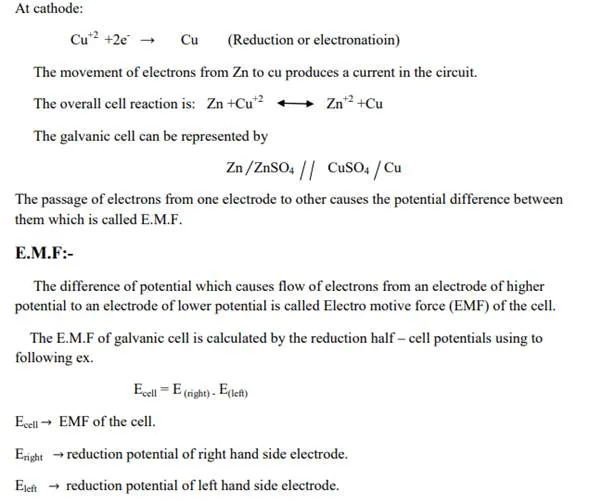
The following reactions take place in the cell.
At cathode


Applications of EMF measurement:-
1. Potentiometric titrations can be carried out.
2. Transport number of ions can be determined.
3. PH can be measured.
4. Hydrolysis const, can be determined. 5. Solubility of sparingly soluble salts can be found.
Differences between Galvanic cell and Electrolytic cell.
