Metallic coatings
The surface of the base metal is coated with another metal (coating metal). Metallic coatings are broadly classified into anodic and cathodic coatings.
1. Anodic coating: the metal used for the surface coating is more anodic than the base metal which is to be protected.
· For example, coating of Al, Cd and Zn on steel surface are anodic because their electrode potentials are lower than that of the base metal iron. Therefore, anodic coatings protect the underlying base metal sacrificially.
· The formation of pores and cracks over the metallic coating exposes the base metal and a galvanic cell is formed between the base metal and coating metal. The coating metal dissolves anodically and the base metal is protected.
2. Cathodic coating:
· Cathodic coatings are obtained by coating a more noble metal (i.e. metals having higher electrode potential like Sn, Au, Ag, Pt etc.) than the base metal. They protect the base metal as they have higher corrosion resistance than the base metal due to cathodic nature.
· Cathodic coating protects the base metal only when the coating is uniform and free from pores.
· The formation of pores over the cathodic coating exposes the base metal (anode) to environment and a galvanic cell is set up. This causes more damage to the base metal.
Methods of application of metallic coatings
1. Hot dipping
· Hot dipping process is applicable to the metals having higher melting point than the coating metal.
· It is carried out by immersing a well cleaned base metal in a bath containing molten coating metal and a flux layer.
· The flux cleans the surface of the base metal and prevents the oxidation of the molten coating metal.
Eg. Coating of Zn, Pb, Al on iron and steel surfaces.
The most widely used hot dipping processes are galvanizing and tinning.
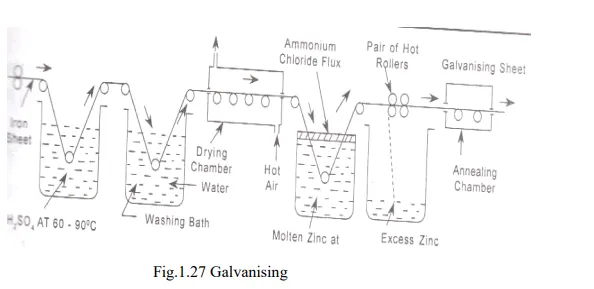
a. Galvanizing
· Galvanizing is a process in which the iron article is protected from corrosion by coating it with a thin layer of zinc.
· It is the anodic protection offered by the zinc. In this process, at first iron or steel is cleaned by pickling with dilute sulphuric acid solution at a temperature range of 60-90oC for 15 to 20 minutes. Therefore, it removes scale, rust and other impurities present and then washed well in a water bath and dried.
· Then after dipped in the bath containing molten zinc which is at 425-450oC. To prevent it from oxide formation, the surface of bath is covered with a ammonium chloride flux. When the iron sheet is taken out it is coated with a thin layer of zinc.
· To remove excess zinc, it is passed through a pair of hot rollers and then it is annealed at a temperature of 450oC followed by cooling.
· Galvanizing is widely used for protecting iron exposed to the atmosphere (roofs, wire fences, pipes etc.) Galvanized metallic sheets are not used for keeping eatables because of the solubility of zinc.

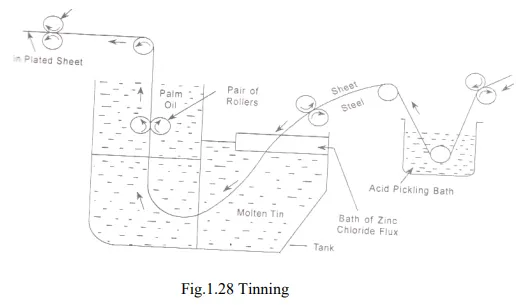
b. Tinning
· The process of coating tin over the iron or steel articles to protect them from undergoing corrosion is known as tinning.
· Tin is a noble metal and therefore it possess more resistance to chemical attack. It is the cathodic protection offered by the tin. In this process, iron sheet is treated in dilute sulphuric acid (pickling) to remove any oxide film, if present.
· A cleaned iron sheet is passed through a bath ZnCl2 molten flux followed by molten tin and finally through a suitable vegetable oil. The ZnCl2 flux helps the molten metal to adhere to the base metallic surface.
· Palm oil protects the tin coated surface against oxidation. Tinning of mild steel plates is done mostly for the requirements of the food stuff industry.
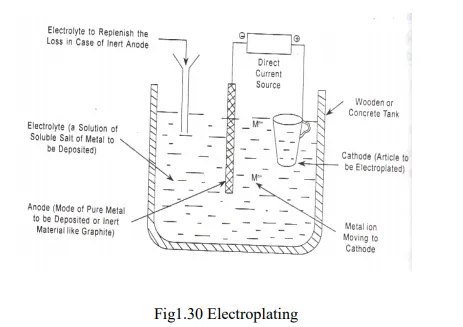
3. Electroplating
· Electroplating is the process of coating metals and protects them from corrosion, wear and chemical attack.
· Electroplating is the method of electro-deposition of metal by means electrolysis over surface of metals and alloys.
· The base metal is first subjected to acid pickling to remove any scales, oxides etc. The base metal is made as cathode of the electrolytic cell and the coating metal is made as anode.
· The two electrolytes are dipped in the electrolyte solution which contains the metal ions to be deposited on the base metal.
· When a direct current is passed from an external source, the coating metal ions migrate towards cathode and get deposited over the surface of base metal in the form of a thin layer.
· Low temperature, medium current density, low metal ion concentration conditions are maintained for better electro-plating.