Corrosion control methods
I. Cathodic protection
The method of protecting the base metal by making it to behave like a cathode is called as cathodic protection.
There are two types of cathodic protection
(a) Sacrificial anode method
(b) Impressed current method.
a. Sacrificial anode method
· In this protection method, the metallic structure to be protected (base metal) is connected by a wire to a more anodic metal so that all the corrosion is concentrated at this more anodic metal.
· The more anodic metal itself gets corroded slowly, while the parent structure (cathodic) is protected. The more active metal so employed is called sacrificial anode. The corroded sacrificial anode is replaced by a fresh one, when consumed completely.
· Metals commonly employed as sacrificial anode are Mg, Zn, Al and their alloys which possess low reduction potential and occupies higher end in electrochemical series.
Eg. A ship-hull which is made up of steel is connected to sacrificial anode (Zn-blocks) which undergoes corrosion leaving the base metal protected.
Eg. The underground water pipelines and water tanks are also protected by sacrificial anode method. By referring to the electrochemical series, the metal with low reduction potential is connected to the base metal which acts as anode.

Fig.1.25 Sacrificial anode method: ship hull and underground water pipeline
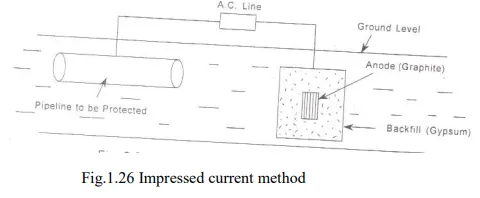
b. Impressed current method
· In this method, an impressed current is applied in opposite direction to nullify the corrosion current, and convert the corroding metal from anode to cathode.
· The impressed current is slightly higher than the corrosion current. Thus the anodic corroding metal becomes cathodic and protected from corrosion.
· The impressed current is taken from a battery or rectified on A.C. line. The impressed current protection method is used for water tanks, water & oil pipe lines, transmission line towers etc.