Electro Chemistry and Corrosion
Introduction
Chemistry is the Study of matter, its properties and the changes it may undergo. All matter is electrical in nature. An atom is made up of sub atomic particles like electors, protons and neutrons etc.
Electro chemistry is a branch of chemistry which deals with the transformation of electrical energy into chemical energy or chemical into electrical energy.
Concept of electrochemistry:
Electrical Conduction:
The substances are divided into 4 types depending upon their capability of flow of electrons.
i) Conductors:
The Substances which allows electricity to pass through them are called conductors.
Ex :- Metals, metal sulphides, acids, alkalis, salt sol. and fused salts
The electrical conductors are of two types.
1. Metallic or Electronic conductors.
2. Electrolytic conductors
ii) Non-conductors:
The substances which do not allow electricity are called non-conductors.
Ex: Pure water, dry wood, rubber, paper, non-metals etc.
iii) Semiconductors:
The substances which partially conduct electricity are called semiconductors. The conducting properties of semi-conducting properties are increased by the addition of certain impurities called “dopping”.
Ex: ‘si’ and addition of V group elements like ‘p’ ‘si’ produces n-type semi-conductor. On addition of iii group element like ‘B’, Al, ‘si’ produces p-type of semi-conductor.
Differences between Metallic Conductors and Electrolytic Conductors

Electrical resistance – ohm’s law.
The current strength flowing through a conductor at uniform temperature is directly proportional to the potential difference applied across to conductor

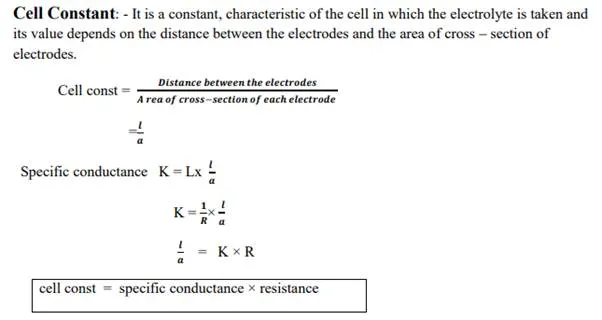
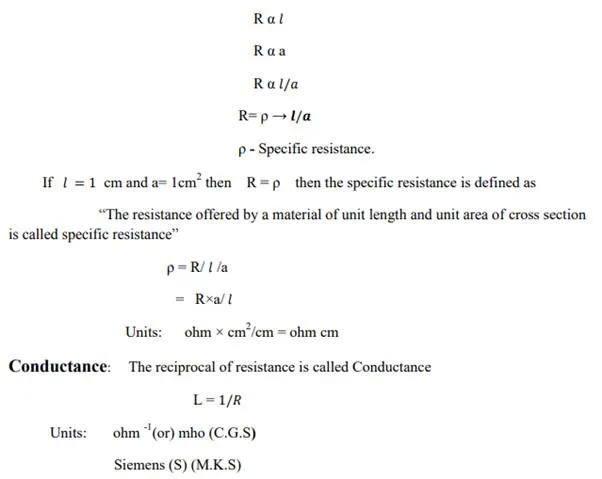
Specific resistance (or) Resistivity:
Ohm found that the solution of electrolyte also offers resistance to flow of current in the solution.
“The resistance (R) of a conductor is directly proportional to its length and inversely proportional to its cross sectional area (a)”