Non-conventional methods of power generation:
Fuel Cells
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent.
Hydrogen produced from the steam methane reforming of natural gas is the most common fuel, but for greater efficiency hydrocarbons can be used directly such as natural gas and alcohols like methanol. Fuel cells are different from batteries in that they require a continuous source of fuel and oxygen/air to sustain the chemical reaction whereas in a battery the chemicals present in the battery react with each other to generate an electromotive (emf). Fuel cells can produce electricity continuously for as long as these inputs are supplied.
The first fuel cells were invented in 1838. The first commercial use of fuel cells came more than a century later in NASA space programs to generate power for probes, satellites and space capsules. Since then, fuel cells have been used in many other applications. Fuel cells are used for primary and backup power for commercial, industrial and residential buildings and in remote or inaccessible areas. They are also used to power fuel-cell vehicles, including forklifts, automobiles, buses, boats, motorcycles and submarines.
There are many types of fuel cells, but they all consist of an anode, a cathode and an electrolyte that allows charges to move between the two sides of the fuel cell. Electrons are drawn from the anode to the cathode through an external circuit, producing direct current electricity. As the main difference among fuel cell types is the electrolyte, fuel cells are classified by the type of electrolyte they use followed by the difference in start-up time ranging from 1 sec for PEMFC to 10 min for SOFC. Fuel cells come in a variety of sizes. Individual fuel cells produce relatively small electrical potentials, about 0.7 volts, so cells are "stacked", or placed in series, to increase the voltage and meet an application's requirements.[2]In addition to electricity, fuel cells produce water, heat and, depending on the fuel source, very small amounts of nitrogen dioxide and other emissions. The energy efficiency of a fuel cell is generally between 40–60%, or up to 85% efficient in cogeneration if waste heat is captured for use.
Types:
Fuel cells come in many varieties; however, they all work in the same general manner. They are made up of three adjacent segments: the anode, the electrolyte, and the cathode. Two chemical reactions occur at the interfaces of the three different segments. The net result of the two reactions is that fuel is consumed, water or carbon dioxide is created, and an electric current is created, which can be used to power electrical devices, normally referred to as the load.
At the anode a catalyst oxidizes the fuel, usually hydrogen, turning the fuel into a positively charged ion and a negatively charged electron. The electrolyte is a substance specifically designed so ions can pass through it, but the electrons cannot. The freed electrons travel through a wire creating the electric current. The ions travel through the electrolyte to the cathode. Once reaching the cathode, the ions are reunited with the electrons and the two react with a third chemical, usually oxygen, to create water or carbon dioxide.
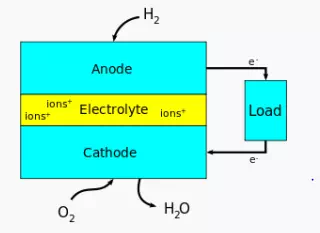
The electrolyte substance usually defines the type of fuel cell.
· The fuel that is used. The most common fuel is hydrogen.
· The anode catalyst breaks down the fuel into electrons and ions. The anode catalyst is usually made up of very fine platinum powder.
· The cathode catalyst turns the ions into the waste chemicals like water or carbon dioxide. The cathode catalyst is often made up of nickel but it can also be a nonmaterial-based catalyst.
A typical fuel cell produces a voltage from 0.6 V to 0.7 V at full rated load. Voltage decreases as current increases, due to several factors:
· Activation loss
· Ohmic loss (voltage drop due to resistance of the cell components and interconnections)
· Mass transport loss (depletion of reactants at catalyst sites under high loads, causing rapid loss of voltage)
To deliver the desired amount of energy, the fuel cells can be combined in series to yield higher voltage, and in parallel to allow a higher current to be supplied. Such a design is called a fuel cell stack. The cell surface area can also be increased, to allow higher current from each cell. Within the stack, reactant gases must be distributed uniformly over each of the cells to maximize the power output.
i) Proton exchange membrane fuel cells (PEMFCs):
In the archetypical hydrogen–oxide proton exchange membrane fuel cell design, a proton-conducting polymer membrane (the electrolyte) separates the anode and cathode sides. This was called a "solid polymer electrolyte fuel cell" (SPEFC) in the early 1970s, before the proton exchange mechanism was well understood.
On the anode side, hydrogen diffuses to the anode catalyst where it later dissociates into protons and electrons. These protons often react with oxidants causing them to become what are commonly referred to as multi-facilitated proton membranes. The protons are conducted through the membrane to the cathode, but the electrons are forced to travel in an external circuit (supplying power) because the membrane is electrically insulating. On the cathode catalyst, oxygen molecules react with the electrons (which have traveled through the external circuit) and protons to form water.
In addition to this pure hydrogen type, there are hydrocarbon fuels for fuel cells, including diesel, methanol and chemical hydrides. The waste products with these types of fuel are carbon dioxide and water, when hydrogen is used the CO2 is released when methane from natural gas is combined with steam in a process called steam methane reforming to produce the hydrogen, this can take place in a different location to the fuel cell potentially allowing the hydrogen fuel cell to be used indoors for example in forklifts.
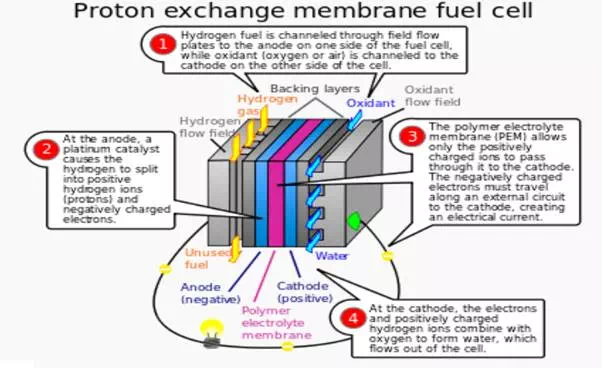
Construction of a high-temperature PEMFC: Bipolar plate as electrode with in-milled gas channel structure, fabricated from conductive composites (enhanced with graphite, carbon black, carbon fiber, and/or carbon annotates for more conductivity) Porous carbon papers; reactive layer, usually on the polymer membrane applied; polymer membrane.
Condensation of water produced by a PEMFC on the air channel wall. The gold wire around the cell ensures the collection of electric current. The different components of a PEMFC are;
1. Bipolar plates,
2. Electrodes,
3. Catalyst,
4. Membrane, and
5. The necessary hardware.
The materials used for different parts of the fuel cells differ by type. The bipolar plates may be made of different types of materials, such as, metal, coated metal, graphite, flexible graphite, C– C composite, carbon–polymer composites etc. The membrane electrode assembly (MEA) is referred as the heart of the PEMFC and is usually made of a proton exchange membrane sandwiched between two catalyst-coated carbon papers. Platinum and/or similar type of noble metals are usually used as the catalyst for PEMFC. The electrolyte could be a polymer membrane.
ii) Phosphoric acid fuel cell (PAFC):
Phosphoric acid fuel cells (PAFC) were first designed and introduced in 1961 by G. V. Elmore and H. A. Tanner. In these cells phosphoric acid is used as a non-conductive electrolyte to pass positive hydrogen ions from the anode to the cathode. These cells commonly work in temperatures of 150 to 200 degrees Celsius. This high temperature will cause heat and energy loss if the heat is not removed and used properly. This heat can be used to produce steam for air conditioning systems or any other thermal energy consuming system.[31] Using this heat incogeneration can enhance the efficiency of phosphoric acid fuel cells from 40– 50% to about 80%.Phosphoric acid, the electrolyte used in PAFCs, is a non-conductive liquid acid which forces electrons to travel from anode to cathode through an external electrical circuit. Since the hydrogen ion production rate on the anode is small, platinum is used as catalyst to increase this ionization rate. A key disadvantage of these cells is the use of an acidic electrolyte. This increases the corrosion or oxidation of components exposed to phosphoric acid.