Primary cells
Primary cells cannot be recharged, that is, the conversion of chemical energy to electrical energy is irreversible and the cell cannot be used once the chemicals are exhausted. Examples of primary cells include the Leclanche cell and the mercury cell. ´
Lechlanche cell ´
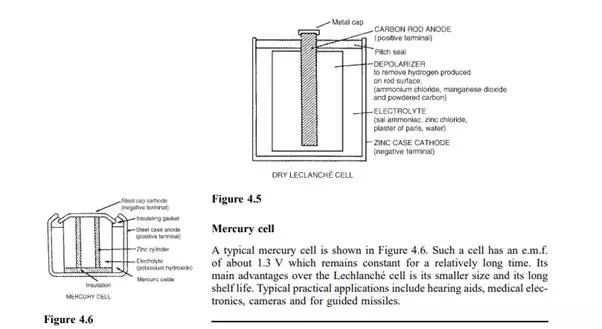
A typical dry Lechlanche cell is shown in Figure 4.5. Such a cell has an ´ e.m.f. of about 1.5 V when new, but this falls rapidly if in continuous use due to polarization. The hydrogen film on the carbon electrode forms faster than can be dissipated by the depolarizer. The Lechlanche cell is ´ suitable only for intermittent use, applications including torches, transistor radios, bells, indicator circuits, gas lighters, controlling switch-gear, and so on. The cell is the most commonly used of primary cells, is cheap, requires little maintenance and has a shelf life of about 2 years.
Secondary cells
Secondary cells can be recharged after use, that is, the conversion of chemical energy to electrical energy is reversible and the cell may be used many times. Examples of secondary cells include the lead-acid cell and the alkaline cell. Practical applications of such cells include car batteries, telephone circuits and for traction purposes — such as milk delivery vans and fork lift trucks.
Lead-acid cell
A typical lead-acid cell is constructed of:
(i) A container made of glass, ebonite or plastic.
(ii) Lead plates
(a) the negative plate (cathode) consists of spongy lead
(b) the positive plate (anode) is formed by pressing lead peroxide into the lead grid.
The plates are interleaved as shown in the plan view of Figure 4.7 to increase their effective cross-sectional area and to minimize internal resistance.
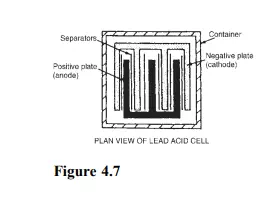
(iii) Separators made of glass, celluloid or wood.
(iv) An electrolyte which is a mixture of sulphuric acid and distilled water.
The relative density (or specific gravity) of a lead-acid cell, which may be measured using a hydrometer, varies between about 1.26 when the cell is fully charged to about 1.19 when discharged. The terminal p.d. of a lead-acid cell is about 2 V.
When a cell supplies current to a load it is said to be discharging. During discharge:
(i) the lead peroxide (positive plate) and the spongy lead (negative plate) are converted into lead sulphate, and
(ii) the oxygen in the lead peroxide combines with hydrogen in the electrolyte to form water. The electrolyte is therefore weakened and the relative density falls.
The terminal p.d. of a lead-acid cell when fully discharged is about 1.8 V.
A cell is charged by connecting a d.c. supply to its terminals, the positive terminal of the cell being connected to the positive terminal of the supply. The charging current flows in the reverse direction to the discharge current and the chemical action is reversed. During charging:
(i) the lead sulphate on the positive and negative plates is converted back to lead peroxide and lead respectively, and
(ii) the water content of the electrolyte decreases as the oxygen released from the electrolyte combines with the lead of the positive plate. The relative density of the electrolyte thus increases.
The colour of the positive plate when fully charged is dark brown and when discharged is light brown. The colour of the negative plate when fully charged is grey and when discharged is light grey.
Alkaline cell
There are two main types of alkaline cell — the nickel-iron cell and the nickel-cadmium cell. In both types the positive plate is made of nickel hydroxide enclosed in finely perforated steel tubes, the resistance being reduced by the addition of pure nickel or graphite. The tubes are assembled into nickel-steel plates. In the nickel-iron cell, (sometimes called the Edison cell or nife cell), the negative plate is made of iron oxide, with the resistance being reduced by a little mercuric oxide, the whole being enclosed in perforated steel tubes and assembled in steel plates. In the nickel-cadmium cell the negative plate is made of cadmium. The electrolyte in each type of cell is a solution of potassium hydroxide which does not undergo any chemical change and thus the quantity can be reduced to a minimum. The plates are separated by insulating rods and assembled in steel containers which are then enclosed in a non-metallic crate to insulate the cells from one another. The average discharge p.d. of an alkaline cell is about 1.2 V.
Advantages of an alkaline cell (for example, a nickel-cadmium cell or a nickel-iron cell) over a lead-acid cell include:
(i) More robust construction
(ii) Capable of withstanding heavy charging and discharging currents without damage
(iii) Has a longer life
(iv) For a given capacity is lighter in weight
(v) Can be left indefinitely in any state of charge or discharge without damage
(vi) Is not self-discharging
Disadvantages of an alkaline cell over a lead-acid cell include:
(i) Is relatively more expensive
(ii) Requires more cells for a given e.m.f.
(iii) Has a higher internal resistance
(iv) Must be kept sealed
(v) Has a lower efficiency
Alkaline cells may be used in extremes of temperature, in conditions where vibration is experienced or where duties require long idle periods or heavy discharge currents. Practical examples include traction and marine work, lighting in railway carriages, military portable radios and for starting diesel and petrol engines. However, the lead-acid cell is the most common one in practical use.